
Concept explainers
a)
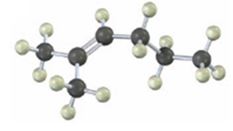
Interpretation:
The product(s) formed when the
Concept introduction:
NBS is used mainly for allylic bromination. An unsymmetrical alkene will lead to a mixture of products as different allyl radicals will be produced as a result of delocalization. The products are not formed in equal amounts because the intermediate allylic radicals are not symmetrical and the reaction at two ends will not take place to the same extent. Reaction at less hindered primary side is more favored.
To give:
The product(s) formed when the alkene shown is treated with NBS.
b)
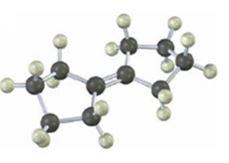
Interpretation:
The product(s) formed when the alkene shown reacts with NBS is/are to be given.
Concept introduction:
NBS is used mainly for allylic bromination. An unsymmetrical alkene will lead to a mixture of products as different allyl radicals will be produced as a result of delocalization. The products are not formed in equal amounts because the intermediate allylic radicals are not symmetrical and the reaction at two ends will not take place to the same extent. Reaction at less hindered primary side is more favored.
To give:
The product(s) formed when the alkene shown is treated with NBS.
Trending nowThis is a popular solution!

Chapter 10 Solutions
Organic Chemistry
- What is the major product of the following reactions? Disregard stereoisomers:arrow_forwardGive the major product(s) of the following reaction. CH3CH20 Give the major product(s) of the following reaction. NaNMe2 Give the major product(s) of the following reaction. ? но. O-arrow_forwardWhich alkene will react with Br₂ to give the following product? Br Brarrow_forward
- Which of the following reactions results in Markovnikov addition of H2O to an alkene?arrow_forwardWhy alkene will yield the most stable carbon cation during the electrophilic addition of HCI. O A OB O O C D O E Ph CF3 D Pharrow_forwardIdentify the alkene product in each of the following Wittig reaction: + Cyclohexyl methyl ketone + (C6H5)3P-CH₂ O 2-Phenylpropene 2-Cyclohexyl-1-phenylpropene 1: O 1,2-Diphenylpropene O 2-Cyclohexylpropenearrow_forward
- 2. Give the major product of the following reactions: =CN OH I-CH3 НО, + Oxyacidarrow_forwardThe following alkene is treated with one equivalent of NBS in CH2Cl2 in the presence of light to give bromination products. Please draw the structural formula for each product formed. Please it’s my last chance so it has to be right. Thank you!arrow_forward4. Give the starting alkene and any other reagents needed to selectively make the following compounds (with no organic byproducts!): OH L Eto OH all OH Br Br Brarrow_forward
- Alkynes undergo many of the same reactions that alkenes do. What organic product(s) would you expect when the following compound is treated with 2 equiv H2, Pd/C?arrow_forwardDetermine the major product of the following reaction: H2 Lindlar's catalystarrow_forwardGive the Major product(s) and name the starting compound with stereochemistry: H2O H2O H2SO4 H,SO, HgSO,arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





