
a)
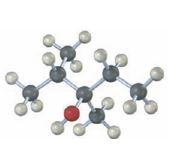
Interpretation:
The structures of
Concept introduction:
The hydroboration reaction takes place with syn stereochemistry and results in a non-Markovnikov addition of water to the double bond in the alkene. The resulting product has the hydroxyl group on the carbon with less number of alkyl substitutents.
The oxymercuration-demercuration reaction takes place with anti stereochemistry and results in Markovnokov addition of water to the double bond in the alkene. The resulting product has the hydroxyl group on the carbon with more number of alkyl substitutents.
To give:
The structures of alkenes that would yield the alcohol shown on hydration.
To state:
Among hydroboration-oxidation and oxymercuration-demercuration methods which method can be used to prepare the alcohol from alkenes.
b)
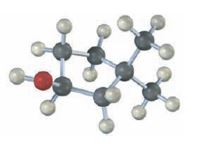
Interpretation:
The structures of alkenes that would yield the alcohol shown on hydration are to be given. Among hydroboration-oxidation and oxymercuration-demercuration, the method which can be used to prepare the alcohol also has to be stated.
Concept introduction:
The hydroboration reaction takes place with syn stereochemistry and results in a non-Markovnikov addition of water to the double bond in the alkene. The resulting product has the hydroxyl group on the carbon with less number of alkyl substitutents.
The oxymercuration-demercuration reaction takes place with anti stereochemistry and results in Markovnokov addition of water to the double bond in the alkene. The resulting product has the hydroxyl group on the carbon with more number of alkyl substitutents.
To give:
The structures of alkenes that would yield the alcohol shown on hydration.
To state:
Among hydroboration-oxidation and oxymercuration-demercuration, the method which can be used to prepare the alcohol.
Trending nowThis is a popular solution!

Chapter 8 Solutions
Organic Chemistry
- Acyl transfer (nucleophilic substitution at carbonyl) reactions proceed in two stages via a "tetrahedral intermediate." Draw the tetrahedral intermediate as it is first formed in the following reaction. CI H₂N OH • You do not have to consider stereochemistry. • Include all valence lone pairs in your answer. • Do not include counter-ions, e.g., Na+, I, in your answer. • In cases where there is more than one answer, just draw one. Sn [Farrow_forwardIf 1-bromopentane is heated in acetone containing NaOH, what is the alkane produced? Draw and explain the step-by-step mechanism of the production of the compoundarrow_forwardCH3 CH3 Br- Br2 .CH3 CH2Cl2 CH3 H3C H3C Br Electrophilic addition of bromine, Brɔ, to alkenes yields a 1,2-dibromoalkane. The reaction proceeds through a cyclic intermediate known as a bromonium ion. The reaction occurs in an anhydrous solvent such as CH,Cl,. In the second step of the reaction, bromide is the nucleophile and attacks at one of the carbons of the bromonium ion to yield the product. Due to steric clashes, the bromide ion always attacks the carbon from the opposite face of the bromonium ion so that a product with anti stereochemistry is formed. Draw curved arrows to show the movement of electrons in this step of the mechanism. Arrow-pushing Instructions CH3 CH3 CH3 CH3 H3C H3C :Br: :Br:arrow_forward
- Draw the major organic product formed by reaction of 2-hexyne with the following reagent: H₂O in H₂SO4 / HgSO4. • Consider E/Z stereochemistry of alkenes. • In cases where there is more than one answer, just draw one. • If no reaction occurs, draw the organic starting material.arrow_forwardDraw the structures of two alkenes that would react to form the haloalkane below upon addition of HBr. Your alkenes should be different regioisomers that yield the haloalkane as the major product without requiring rearrangement to occur.arrow_forwardDraw the overall reaction of 2-bromo-2-methylpropane with H2O to give the alcohol product. Include all by-products that form.arrow_forward
- Acyl transfer (nucleophilic substitution at carbonyl) reactions proceed in two stages via a "tetrahedral intermediate." Draw the tetrahedral intermediate as it is first formed in the following reaction. 0 OH CI + H₂N • You do not have to consider stereochemistry. • Include all valence lone pairs in your answer. • Do not include counter-ions, e.g., Na+, I, in your answer. In cases where there is more than one answer, just draw one. A ChemDoodle Activate Windowsarrow_forwardBy taking into account electronegativity differences, draw the products formed by heterolysis of the carbon–heteroatom bond in each molecule. Classify the organic reactive intermediate as a carbocation or a carbanion.arrow_forwardA problem often encountered in the oxidation of primary alcohols to acids is that esters are sometimes produced as by-products. For example, oxidation of ethanol yields acetic acid and ethyl acetate: Propose a mechanism to account for the formation of ethyl acetate. Take into account the reversible reaction between aldehydes and alcohols:arrow_forward
- Create a hydrohalogenation reaction using a 5 carbon alkene illustrating Markovnikov's rule. Draw and name all reagentsarrow_forwardDescribe how 1-ethylcyclohexanol can be prepared from cyclohexane. You can use any inorganic reagents, any solvents, and any organic reagents as long as they contain no more than two carbons.arrow_forwardDraw the alkene that would react with the reagent given to account for the product formed. CH3 HCI CH3 CHCCH, ? + Či CH3 You do not have to consider stereochemistry. • You do not have to explicitly draw H atoms. • In cases where there is more than one answer, just draw one.arrow_forward
