
Concept explainers
a)
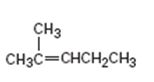
Interpretation:
The product obtained by the catalytic hydrogenation of 2-methyl-2-pentene is to be given.
Concept introduction:
To give:
The product obtained by the catalytic hydrogenation of 2-methyl-2-pentene.
b)
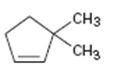
Interpretation:
The product obtained by the catalytic hydrogenation of 3,3-dimethylcyclopentene is to be given.
Concept introduction:
Alkenes react with H2 in the presence of metal catalysts such as palladium or platinum to yield the corresponding alkanes as the products. The addition occurs with syn stereochemistry. Normally only a single product is produced as the catalyst will interact only from the least hindered and most accessible face of the alkene.
To give:
The product obtained by the catalytic hydrogenation of 3,3-dimethylcyclopentene.
c)
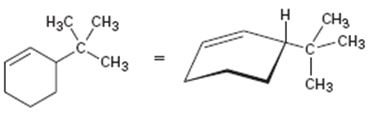
Interpretation:
The product obtained by the catalytic hydrogenation of 3-tert-butylcyclohexene is to be given.
Concept introduction:
Alkenes react with H2 in the presence of metal catalysts such as palladium or platinum to yield the corresponding alkanes as the products. The addition occurs with syn stereochemistry. Normally only a single product is produced as the catalyst will interact only from the least hindered and most accessible face of the alkene.
To give:
The product obtained by the catalytic hydrogenation of 3-tert-butylcyclohexene.
Trending nowThis is a popular solution!

Chapter 8 Solutions
Organic Chemistry
- When the given reactions below are done once, which of the following reactions is used to prepare dihalogenated alkyl halides as major products? Select one: O Reaction of ethane with Cl₂ in the presence of light Reaction of ethene with HBr Reaction of ethyne with two moles of HCI Reaction of ethene with Br₂ in H₂Oarrow_forwardWhat products would you expect from reaction of the following alkenes with NBS? If more than one product is formed, show the structures of all.arrow_forwardWhat alkene with the molecular formula C6H12, when treated with ozone and then dimethyl sulfide, gives the following product(s)?arrow_forward
- 3-Hexanone can be synthesized from butanal and ethane using a Grignard reaction. The first step must be O creation of a carbocation O protonation of an oxygen nucleophilic attack of the aldehyde free radical halogenation preparation of an acetylidearrow_forwardHow many alkenes yield 2,3−dimethylbutane on catalytic hydrogenation?arrow_forwardWrite the expected substitution product(s) for each reaction and predict the mechanism by which each product is formedarrow_forward
- One compound that contributes to the “seashore smell” at beaches in Hawai‘i is dictyopterene D', a component of a brown edible seaweed called limu lipoa. Hydrogenation of dictyopterene D' with excess H2 in the presence of a Pd catalyst forms butylcycloheptane. Ozonolysis with O3 followed by (CH3)2S forms CH2(CHO)2, HCOCH2CH(CHO)2, and CH3CH2CHO. What are possible structures of dictyopterene D'?arrow_forwardPredict the products of the following reactions. Include stereochemistry when necessary. For reactions with more than one step show the product formed after each step.arrow_forwardQuestão 10A certain hydrocarbon had the molecular formula C16H26 and contained two triple bonds. Ozonolysis resulted in CH3(CH2)4CO2H and HO2CCH2CH2CO2H as the only products. What is the reasonable structure for this hydrocarbon? Hexadec-6,10-dino undec-1,5-dino Hept-1,5-dino hex-1,5-dino naharrow_forward
- what product would you expect from reaction of cyclohexane with hbr with hclarrow_forwardPredict the products of the following reaction. Include stereochemistry when necessary. For reactions with more than one step show the product formed after each step.arrow_forwardWhich represents an efficient synthetic route to go from an alkane to an alkene? O elimination with NaNH2, followed by a water workup O anti-Markovnikov hydrohalogenation, followed by elimination O radical bromination, followed by elimination O hydration, followed by elimination O hydration, followed by ozonolysis of the double bondarrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
