
Concept explainers
Interpretation:
Using octadecanoic (stearic) acid and any necessary organic and inorganic reagents, an efficient synthesis for each compound is to be described.
Concept introduction:
The reduction of
The primary alcohol on oxidation with pyridinium dichromate
In Clemmensen reduction, the carbonyl group (aldehyde or
The esters can be synthesized by acid catalyzed condensation of carboxylic acid with alcohol.
The ester on reaction with one molar equivalent of Grignard’s reagent in diethyl ether gives ketone by carbon-carbon bond formation.
The ester on reaction with two molar equivalents of Grignard’s reagent in diethyl ether gives tertiary alcohol.
The dehydration of alcohol is the loss of
The alcohol on acid catalyzed dehydration gives corresponding alkene.
The alkene on hydrogenation with the catalyst undergoes addition of hydrogen across the double bond and forms an alkane.
The primary amine can be prepared by the acylation of ammonia.
The secondary amide can be prepared by the nucleophilic substitution of acyl chloride by amine. The two moles amines used with one mole of acyl chloride, because one amine molecule acts as a nucleophile and second acts as a Brønsted base.
The carboxylic acids on reaction with thionyl chloride forms acyl chloride by replacing the hydroxyl group of carboxylic acid with chlorine atom.
The primary amide on reduction with lithium aluminum hydride
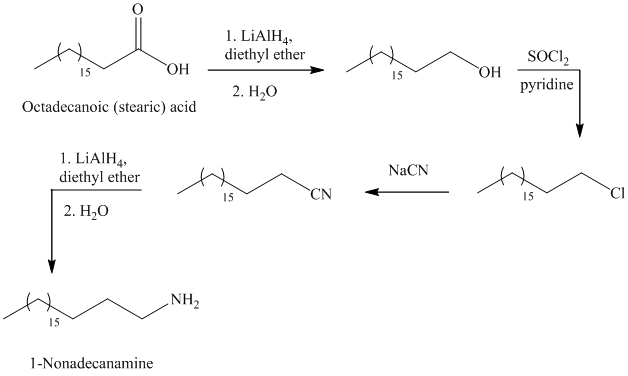
The reaction of thionyl chloride with alcohol gives alkyl halide.
The reaction of alkyl halide with sodium cyanide gives alkyl cyanide.
The cyanide (nitrile) can be reduced to primary amine using lithium aluminum hydride
The alkyl bromide can be prepared by the reaction of alcohol with phosphorus tribromide
Grignard reagents are prepared by the reaction of the magnesium metal with an alkyl or aryl halide usually in diethyl ether as the solvent.
The potassium or sodium dichromate in presence of strong acid forms chromic acid which is a good oxidizing agent, in hydrous medium oxidizes primary alcohol to carboxylic acid.
The epoxide on treatment with Grignard reagent undergoes epoxide ring opening by forming a corresponding alcohol.
Answer to Problem 28P
Solution:
a)
b)
c)
d) 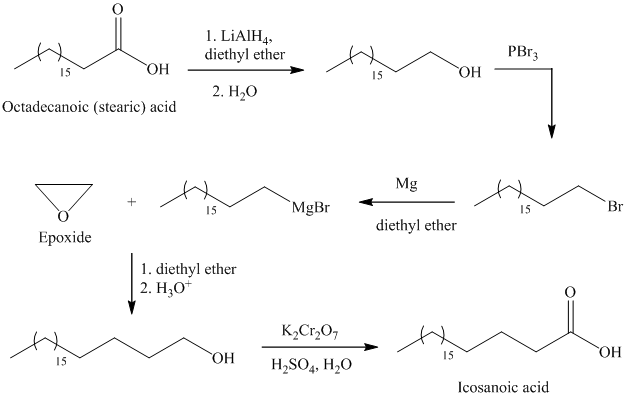
e) 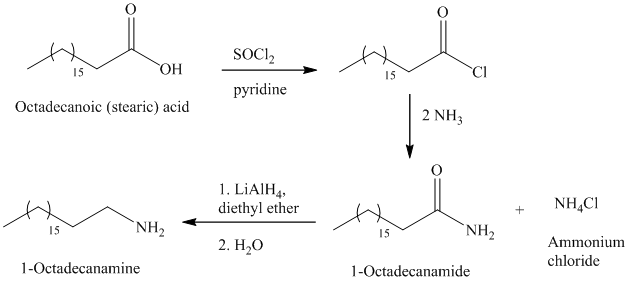
f)
Explanation of Solution
The structure of octadecanoic (stearic) acid is shown below:
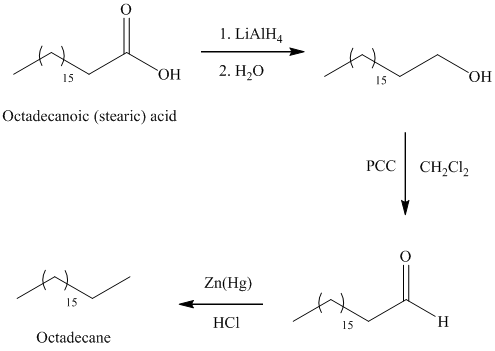
a) Octadecane
The synthesis of octadecane from octadecanoic acid can be done by following reactions sequence:
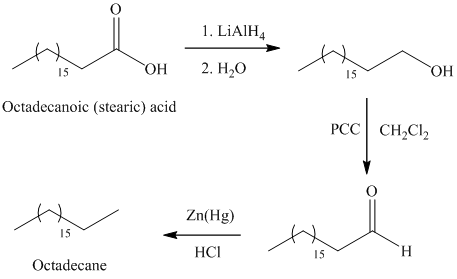
The octadecanoic acid on reaction with lithium aluminum hydride in aqueous medium reduced to octadecanol which is further on oxidation with pyridinium dichromate
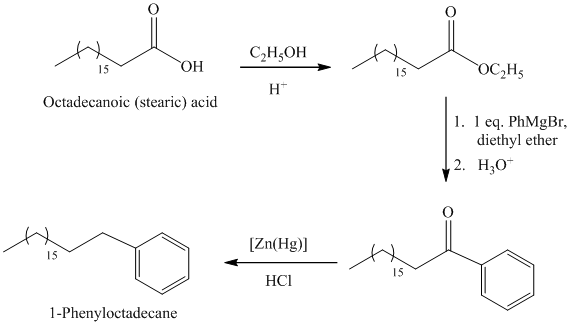
b)
The synthesis of
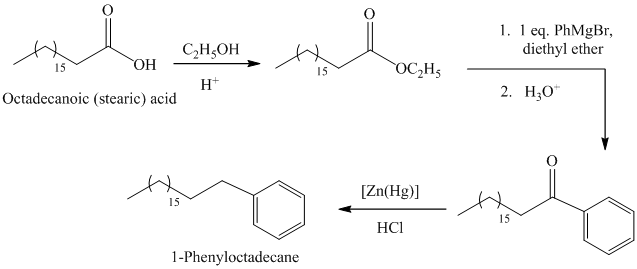
The octadecanoic acid first converted to an ester by reacting it with ethanol in acidic condition. The ester formed is then reacted with a Grignard’s reagent phenylmagnesium bromide
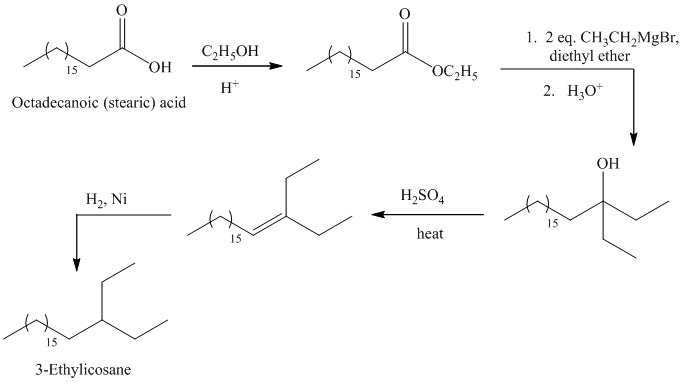
c)
The synthesis of
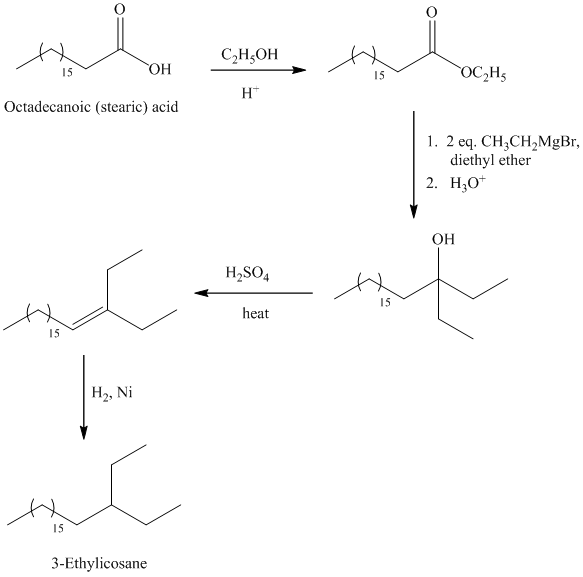
The octadecanoic acid is first converted to an ester by reacting it with ethanol in acidic condition. The ester formed is then reacted with a Grignard’s reagent ethyl bromide bromide
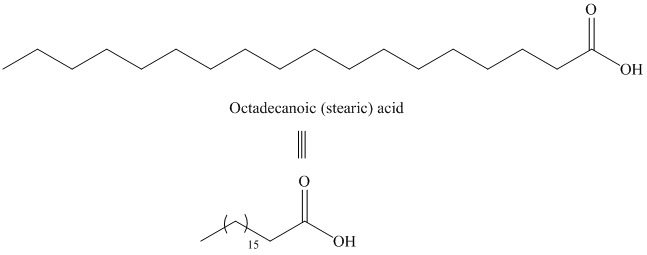
d) Icosanoic acid
The synthesis of Icosanoic acid from octadecanoic acid can be done by following reactions sequence:
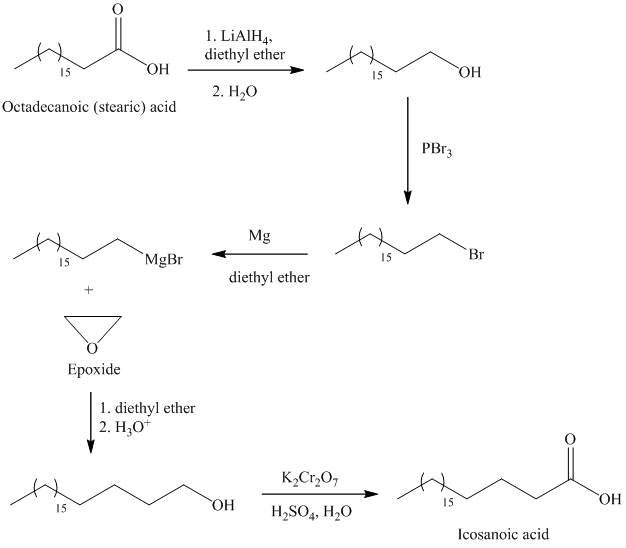
In the first step, the octadecanoic acid is reduced to primary alcohol by reducing agent lithium aluminum hydride
e)
The synthesis of
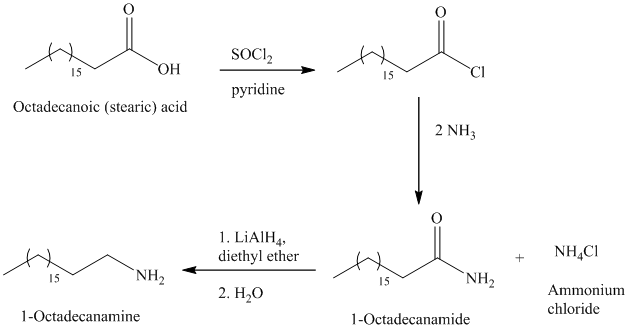
The octadecanoic acid on reaction with thonyl chloride in presence of pyridine gave the product of acyl chloride. The acyl chloride is converted to
f)
The synthesis of
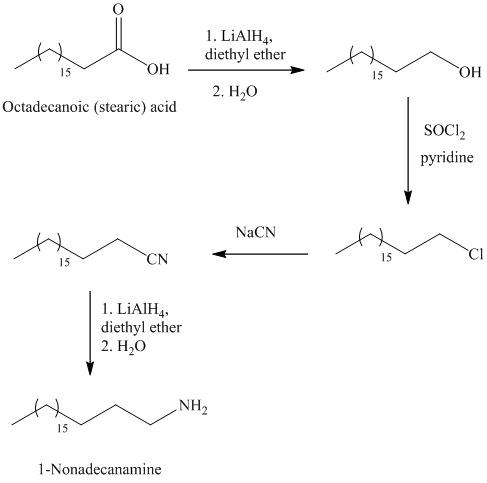
In the first step, the octadecanoic acid is reduced to primary alcohol by reducing agent lithium aluminum hydride
Want to see more full solutions like this?
Chapter 25 Solutions
Organic Chemistry - Standalone book
- Ethyl butyrate, CH3CH2CH2CO2CH2CH3, is an artificial fruit flavor commonly used in the food industry for such flavors as orange and pineapple. Its fragrance and taste are often associated with fresh orange juice, and thus it is most commonly used as orange flavoring.It can be produced by the reaction of butanoic acid with ethanol in the presence of an acid catalyst (H+): CH3CH2CH2CO2H(l)+CH2CH3OH(l)H+⟶CH3CH2CH2CO2CH2CH3(l)+H2O(l) a) Given 7.70 g of butanoic acid and excess ethanol, how many grams of ethyl butyrate would be synthesized, assuming a complete 100% yield? b) A chemist ran the reaction and obtained 5.25 g of ethyl butyrate. What was the percent yield? c) The chemist discovers a more efficient catalyst that can produce ethyl butyrate with a 78.0% yield. How many grams would be produced from 7.70 g of butanoic acid and excess ethanol?arrow_forwardEthyl butyrate, CH3CH2CH2CO2CH2CH3, is an artificial fruit flavor commonly used in the food industry for such flavors as orange and pineapple. Its fragrance and taste are often associated with fresh orange juice, and thus it is most commonly used as orange flavoring.It can be produced by the reaction of butanoic acid with ethanol in the presence of an acid catalyst (H+): CH3CH2CH2CO2H(l)+CH2CH3OH(l)H+⟶CH3CH2CH2CO2CH2CH3(l)+H2O(l). The chemist discovers a more efficient catalyst that can produce ethyl butyrate with a 78.0% yield. How many grams would be produced from 8.50 gof butanoic acid and excess ethanol? Express your answer in grams to three significant figures.arrow_forwardEthyl butyrate, CH3CH2CH2CO2CH2CH3, is an artificial fruit flavor commonly used in the food industry for such flavors as orange and pineapple. Its fragrance and taste are often associated with fresh orange juice, and thus it is most commonly used as orange flavoring.It can be produced by the reaction of butanoic acid with ethanol in the presence of an acid catalyst (H+): CH3CH2CH2CO2H(l)+CH2CH3OH(l)H+⟶CH3CH2CH2CO2CH2CH3(l)+H2O(l) Given 8.50 g of butanoic acid and excess ethanol, how many grams of ethyl butyrate would be synthesized, assuming a complete 100%yield? Express your answer in grams to three significant figures.arrow_forward
- Esterification of Carboxylic Acids Provide the reactions (chemical equations) on esterification of the following acids: Ethanoic acid Butanoic acid Benzoic acid Acetic acid + methanol Acetic acid + ethanol Salicylic acid + methanol Acetic acid + benzyl alcoholarrow_forwardIn preparation of dibenzalacetone, what products are prepared?arrow_forwardFormalin Benzene Salicylic acid Acetone Anisole what compounds are carbonyl-containing compounds and non-carbonyl-containing compoundsarrow_forward
- Predict the products formed when cyclohexanone reacts with the following reagents.phenylhydrazine and weak acidarrow_forwardSaponification product of butylpropanoate is: Propanol and Sodium butanoate Butanol and sodium propanoate Butanol and Propanoic acid Butanoic acid and Propanoic acidarrow_forwardGive IUPAC names for the following acyl derivatives:arrow_forward
- Fenfluramine and phentermine are two components of fen–phen, an appetite suppressant withdrawn from the market in 1997 after it was shown to damage the heart valves in some patients. What products are formed when fenfluramine and phentermine are each treated with acetic acid (CH3CO2H)?arrow_forwardGive the IUPAC name of the product formed in the following reaction of ethyl cyclopentanecarboxylate with sodium hydroxide. NaOH H2Oarrow_forward
 Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning





