
Concept explainers
(a)
Interpretation:
The non-hydrogen atom from the given species having a complete octet is to be stated. The formal charge on the atom is to be stated.
Concept introduction:
Chemical compounds contain two types of bonds. These are known as ionic and covalent bonds. In ionic bonds, the ions are held by the electrostatic interaction between them. In covalent bonds, the atoms are held together by the sharing of electrons. The formal charge is the charge on the constituent atoms in a molecule. It is calculated by using valence electrons of the atom.
Answer to Problem 1.22AP
The non-hydrogen atom in
Explanation of Solution
The structure of
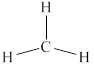
Figure 1
From the above figure, it is clear that number of electrons around carbon atom is
The valence electrons of carbon atom in the
Thus, the formal charge on carbon atom in
The non-hydrogen atom in
(b)
Interpretation:
The non-hydrogen atom from the given species having a complete octet is to be stated. The formal charge on the atom is to be stated.
Concept introduction:
Chemical compounds contain two types of bonds. These are known as ionic and covalent bonds. In ionic bonds, the ions are held by the electrostatic interaction between them. In covalent bonds, the atoms are held together by the sharing of electrons. The formal charge is the charge on the constituent atoms in a molecule. It is calculated by using valence electrons of the atom.
Answer to Problem 1.22AP
The non-hydrogen atom in
Explanation of Solution
The structure of
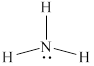
Figure 2
From the above figure, it is clear that number of electrons around nitrogen atom is
The valence electrons of nitrogen atom in the
Thus, the formal charge on nitrogen atom in
The non-hydrogen atom in
(c)
Interpretation:
The non-hydrogen atom from the given species having a complete octet is to be stated. The formal charge on the atom is to be stated.
Concept introduction:
Chemical compounds contain two types of bonds. These are known as ionic and covalent bonds. In ionic bonds, the ions are held by the electrostatic interaction between them. In covalent bonds, the atoms are held together by the sharing of electrons. The formal charge is the charge on the constituent atoms in a molecule. It is calculated by using valence electrons of the atom.
Answer to Problem 1.22AP
The non-hydrogen atom in
Explanation of Solution
The structure of

Figure 3
From the above figure, it is clear that number of electrons around carbon atom is
The valence electrons of carbon atom in the
Thus, the formal charge on carbon atom in
The non-hydrogen atom in
(d)
Interpretation:
The non-hydrogen atom from the given species having a complete octet is to be stated. The formal charge on the atom is to be stated.
Concept introduction:
Chemical compounds contain two types of bonds. These are known as ionic and covalent bonds. In ionic bonds, the ions are held by the electrostatic interaction between them. In covalent bonds, the atoms are held together by the sharing of electrons. The formal charge is the charge on the constituent atoms in a molecule. It is calculated by using valence electrons of the atom.
Answer to Problem 1.22AP
The non-hydrogen atom in
Explanation of Solution
The structure of
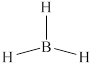
Figure 4
From the above figure, it is clear that number of electrons around boron atom is
The valence electrons of boron atom in the
Thus, the formal charge on boron atom in
The non-hydrogen atom in
(e)
Interpretation:
The non-hydrogen atom from the given species having a complete octet is to be stated. The formal charge on the atom is to be stated.
Concept introduction:
Chemical compounds contain two types of bonds. These are known as ionic and covalent bonds. In ionic bonds, the ions are held by the electrostatic interaction between them. In covalent bonds, the atoms are held together by the sharing of electrons. The formal charge is the charge on the constituent atoms in a molecule. It is calculated by using valence electrons of the atom.
Answer to Problem 1.22AP
The non-hydrogen atom in
Explanation of Solution
The structure of
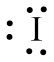
Figure 5
From the above figure, it is clear that number of electrons around iodine atom is
The valence electrons of iodine atom in the
Thus, the formal charge on iodine atom in
The non-hydrogen atom in
(f)
Interpretation:
The non-hydrogen atom from the given species having a complete octet is to be stated. The formal charge on the atom is to be stated.
Concept introduction:
Chemical compounds contain two types of bonds. These are known as ionic and covalent bonds. In ionic bonds, the ions are held by the electrostatic interaction between them. In covalent bonds, the atoms are held together by the sharing of electrons. The formal charge is the charge on the constituent atoms in a molecule. It is calculated by using valence electrons of the atom.
Answer to Problem 1.22AP
The non-hydrogen atom in
Explanation of Solution
The structure of
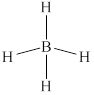
Figure 6
From the above figure, it is clear that number of electrons around boron atom is
The valence electrons of boron atom in the
Thus, the formal charge on boron atom in
The non-hydrogen atom in
Want to see more full solutions like this?
Chapter 1 Solutions
Organic Chemistry
- Write all resonance structures of chlorobenzene, C6H5Cl, a molecule with the same cyclic structure as benzene. In all structures, keep the CCl bond as a single bond. Which resonance structures are the most important?arrow_forwardGiven the bonds C N, C H, C Br, and S O, (a) which atom in each is the more electronegative? (b) which of these bonds is the most polar?arrow_forwardDraw Lewis structures for HFO4, HFO3, HC0O4, HC0O3, HCO2. (These molecules have the halogen atom as the central atom. All O atoms are attached to the halogen. The hydrogen atom is bonded to one of the O atoms.) Use formal charges to determine which molecule is least likely to occur in nature. (A) HFO4 (B) HC(O2 (C) HC!O3 (D) HFO3 (E) HC\O4 DO000arrow_forward
- Calculate the formal charge of each element in the following compounds and ions:(a) F2CO(b) NO–(c) BF4−(d) SnCl3−(e) H2CCH2(f) ClF3(g) SeF6(h) PO43−arrow_forward(a) Draw Lewis structure of the following: (i) H3PO4 (ii) N3 - (b) How many hydrogen atoms are present in 10 g of sugar? (c) Calculate formal charge of each atom of CH3O-arrow_forward(a) Determine the formal charge of oxygen in the following structure. If the atom is formally neutral, indicate a charge of zero. (b) Draw an alternative Lewis (resonance) structure for the compound given in part (a). Show the unshared pairs and nonzero formal charges in your structure. Don't use radicals. Formal charge on O 0arrow_forward
- A stable triatomic molecule can be formed that contains one atom each of nitrogen, sulfur, and fluorine. Three bonding structures are possible, depending on which is the central atom: NSF, SNF, and SFN. (a) Write a Lewis diagram for each of these molecules, indicating the formal charge on each atom. (b) Often, the structure with the least separation of formal charge is the most stable. Is this statement consistent with the observed structure for this molecule— namely, NSF, which has a central sulfur atom? (c) Does consideration of the electronegativities of N, S, and F from Figure 3.18 help rationalize this observed structure? Explain. 100. The gasarrow_forwardWrite Lewis structures for these compounds. Show all valence electrons. None of them contains a ring of atoms. (a) Hydrogen peroxide, H2O2 (b) Hydrazine, N2H4 (c) Methanol, CH3OHarrow_forwardCyanogen (CN)2 is known as pseodohalogen because it has some properties like halogens. It is composed of two CN’s joined together.(i) Draw the Lewis structure for all the possible combination for (CN)2.(ii) Calculate the formal charge and determine which one of the structures that you have drawn is most stable.(iii) For the stable structure, determine the geometry around the two central atoms.(iv) For the stable structure, draw the dipole arrows for the bonds.(v) Base on the stable structure, determine the polarity of molecule and state your reason.arrow_forward
- Draw resonance structures for the bicarbonate ion, HCO3-. (a) Does HCO3- have the same number of resonance structures as the CO32- ion? Are any less likely than others? (b) What are the formal charges on the O and C atoms in HCO3- ? What is the average formal charge on the O atoms? Compare this with the O atoms in CO32- . (c) Protonation of HCO3- gives H2CO3. How do formal charges predict where the H+ ion will be attached?arrow_forwardDraw all possible resonance structures for each of these compounds. Determine the formal charge on each atom in each of the resonance structures:(a) O3(b) SO2(c) NO2 −(d) NO3−arrow_forward(a) Describe the molecule xenon trioxide, XeO3, using four possible Lewis structures, one each with zero, one, two, or three Xe—O double bonds. (b) Do any of these resonance structures satisfy the octet rule for every atom in the molecule? (c) Do any of the four Lewis structures have multiple resonance structures? If so, how many resonance structures do you find? (d) Which of the Lewis structures in (a) yields the most favorable formal charges for the molecule?arrow_forward
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning

