
(a)
Interpretation:
It is to be determined whether the given solvent is suitable for a reaction involving
Concept introduction:
Leveling effects refers to the effect of a solvent on the behavior of acids and bases. If the reactant is a very strong acid or base, it can react with the solvent in an undesired proton transfer reaction. At equilibrium, the strongest acid that can occur in solution is the protonated solvent, and the strongest base that can occur in solution is the deprotonated solvent. For the leveling effect, a solvent is unsuitable for a particular reactant if the reactant (lower
Answer to Problem 6.9P
With respect to the leveling effect, water is not a suitable solvent for a reaction involving
Explanation of Solution
The reaction of
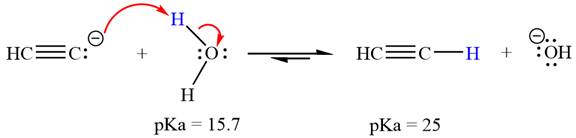
Water,
The solvent effect on the reactant is determined with respect to the leveling effect.
(b)
Interpretation:
It is to be determined whether the given solvent is suitable for a reaction involving
Concept introduction:
Leveling effects refers to the effect of a solvent on the behavior of acids and bases. If the reactant is a very strong acid or base, it can react with the solvent in an undesired proton transfer reaction. At equilibrium, the strongest acid that can occur in solution is the protonated solvent, and the strongest base that can occur in solution is the deprotonated solvent. For the leveling effect, a solvent is unsuitable for a particular reactant if the reactant (lower
Answer to Problem 6.9P
With respect to the leveling effect, ethanol is not a suitable solvent for a reaction involving
Explanation of Solution
The reaction of

Ethanol,
The solvent effect on the reactant is determined with respect to the leveling effect.
(c)
Interpretation:
It is to be determined whether the given solvent is suitable for a reaction involving
Concept introduction:
Leveling effects refers to the effect of a solvent on the behavior of acids and bases. If the reactant is a very strong acid or base, it can react with the solvent in an undesired proton transfer reaction. At equilibrium, the strongest acid that can occur in solution is the protonated solvent, and the strongest base that can occur in solution is the deprotonated solvent. For the leveling effect, a solvent is unsuitable for a particular reactant if the reactant (lower
Answer to Problem 6.9P
With respect to the leveling effect, ethanamide is not a suitable solvent for a reaction involving
Explanation of Solution
The reaction of
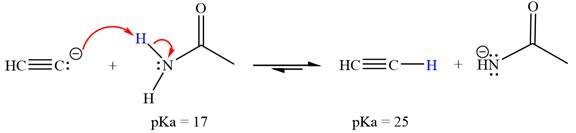
Ethanamide,
The solvent effect on the reactant is determined with respect to the leveling effect.
(d)
Interpretation:
It is to be determined whether the given solvent is suitable for a reaction involving
Concept introduction:
Leveling effects refers to the effect of a solvent on the behavior of acids and bases. If the reactant is a very strong acid or base, it can react with the solvent in an undesired proton transfer reaction. At equilibrium, the strongest acid that can occur in solution is the protonated solvent, and the strongest base that can occur in solution is the deprotonated solvent. For the leveling effect, a solvent is unsuitable for a particular reactant if the reactant (lower
Answer to Problem 6.9P
With respect to the leveling effect,
Explanation of Solution
The reaction of
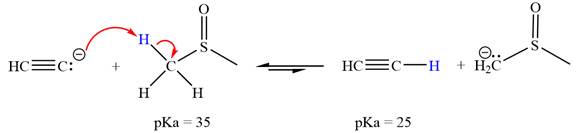
Acetylene,
The solvent effect on the reactant is determined with respect to the leveling effect.
(e)
Interpretation:
It is to be determined whether the given solvent is suitable for a reaction involving
Concept introduction:
Leveling effects refers to the effect of a solvent on the behavior of acids and bases. If the reactant is a very strong acid or base, it can react with the solvent in an undesired proton transfer reaction. At equilibrium, the strongest acid that can occur in solution is the protonated solvent, and the strongest base that can occur in solution is the deprotonated solvent. For the leveling effect, a solvent is unsuitable for a particular reactant if the reactant (lower
Answer to Problem 6.9P
With respect to the leveling effect,
Explanation of Solution
The reaction of
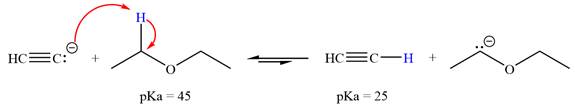
Acetylene,
The solvent effect on the reactant is determined with respect to the leveling effect.
Want to see more full solutions like this?
Chapter 6 Solutions
Organic Chemistry: Principles and Mechanisms (Second Edition)
- Acid-Base Equilibria Many factors contribute to the acidity of organic compounds. Electronegativity, resonance, induction, hybridization, aromaticity, and atomic size, all play a role. In the following comparisons, you are asked to identify the factor(s) that would be most important to analyze when predicting relative acidity, and then to predict the trend in acidity and pKa values. For each of the following pairs of compounds answer the following two multiple-choice questions. 1. What factor(s) are the most important to consider when predicting the relative acidity of the two compounds? a. Electronegativity of the atom possessing the hydrogen. b. Resonance stabilization of the anionic conjugate base. c. Inductive stabilization of the anionic conjugate base. d. Hybridization of the atom possessing the hydrogen. e. The atomic size of the atom possessing the hydrogen.arrow_forwardplease make the explanation clear and thank you for your helparrow_forwardLow-molecular-weight dicarboxylic acids normally exhibit two different pK, values. Ionization of the first carboxyl group is easier than the second. This effect diminishes with molecular size, and for adipic acid and longer chain dicarboxylic acids, the two acid ionization constants differ by about one pK unit. Dicarboxylic Acid Structural Formula pK,2 a1 Охalic НООССООН 1.23 4.19 Malonic НООСCH,COOH 2.83 5.69 Succinic HOOC(CH,),COOH 4.16 5.61 Glutaric HOOC(CH,),COOH 4.31 5.41 Adipic HOOC(CH,),COOH 4.43 5.41 Why do the two pK, values differ more for the shorter chain dicarboxylic acids than for the longer chain dicarboxylic acids?arrow_forward
- 4. The pk, of vanillin is about 9, which is much more acidic than a normal alcohol. Draw a reaction showing the deprotonation of vanillin with NaOH, and then draw six resonance structures of the conjugate base. Draw the hybrid structure and clearly indicate how the negative charge is distributed in the compound.arrow_forwardYou are planning to carry out a reaction that produces protons. The reaction will be buffered at pH = 10.5. Would it be better to use a protonated methylamine/methylamine buffer or a protonated ethylamine/ethylamine buffer? (pKa of protonated methylamine = 10.7; pKa of protonated ethylamine = 11.0)arrow_forwardArrange the following compounds in the increasing order of their acid strength: p-cresol, p-nitrophenol, phenolarrow_forward
- Which of the following will result to the formation of a deep red solution. * removal of ferric chloride removal of ammonium thiocyanate addition of ammonium thiocyanate addition of ammonium chloridearrow_forwardGiven the fact that ammonia is a weak base, should the extraction be carried out at a high or low pH to maximise the separation. Explain your reasoning in detail.arrow_forwardPhenol (hydroxybenzene) behaves as a weak acid. a) Write out the equilibrium equation for its partial dissociation in water. b) Write out the expression for the acid dissociation constant, Ka. d) Draw the conjugate base of phenol and show how it is stabilised by resonance. e) Compare and explain the acidity of phenol (p = 9.9) with that of: cyclohexanol (pk = 16.0) 3-fluorophenol (pK₁ = 9.3) 4-acetylphenol (pK, = 8.1)arrow_forward
- Will acetylene react with sodium hydride according to the following equation to form a salt and hydrogen, H2? Using pKa values given in Table 4.1, calculate Keq for this equilibrium.arrow_forward16) Identify the following: (a) The Lux-Flood acid in the reaction below is 2Fe³+ + 3CO3 →→ Fe₂O3 + 3C0₂ - (b) The Usanovich base in the reaction below is 6Cl + Cr₂0₂² + 14H* → 3Cl₂ + 2Cr³+ + 7H₂O (c) The solvent system base in the reaction below is HNO3 + 2H₂SO4 →NO₂* + H3O* + 2HSO,”arrow_forwardUsing your knowledge on structural effects, account for the following observations by giving appropriate explanations. 1) Boron trifluoride (BF3) is a stronger Lewis acid than trimethyl borate [(CH3O)3B]. 2) Piperidine is a much stronger Lewis base than pyridine.arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning


