
a)
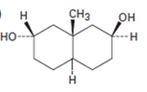
Interpretation:
The product formed when the
Concept introduction:
If the two cyclohexane rings are fused in trans manner the two hydrogens at the ring junction will be diaxial. If the ring fusion is cis, then one hydrogen at the ring junction is axial while that in the other junction is equatorial.
Among the disubstituted cyclohexanes, 1,2- cis, 1,3- trans and 1,4-cis substituents will have axial and equatorial arrangements. 1,2-trans, 1,3-cis and 1,4-trans substituents will have either diequatorial or diaxial arrangements.
Equatorial –OH gets easily acetylated because it is free from steric strains.
To give:
The product formed when the diol shown reacts with one equivalent of acetic anhydride.
b)
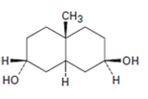
Interpretation:
The product formed when the diol shown reacts with one equivalent of acetic anhydride is to be given.
Concept introduction:
If the two cyclohexane rings are fused in trans manner the two hydrogens at the ring junction will be diaxial. If the ring fusion is cis, then one hydrogen at the ring junction is axial while that in the other junction is equatorial.
Among the disubstituted cyclohexanes, 1,2- cis, 1,3- trans and 1,4-cis substituents will have axial and equatorial arrangements. 1,2-trans, 1,3-cis and 1,4-trans substituents will have either diequatorial or diaxial arrangements.
Equatorial –OH gets easily acetylated because it is free from steric strains.
To give:
The product formed when the diol shown reacts with one equivalent of acetic anhydride.
Trending nowThis is a popular solution!

Chapter 27 Solutions
Organic Chemistry
- Aldehydes and ketones react with one molecule of an alcohol to form compounds called hemiacetals, in which there is one hydroxyl group and one ether-like group. Reaction of a hemiacetal with a second molecule of alcohol gives an acetal and a molecule of water. We study this reaction in Chapter 16. Draw structural formulas for the hemiacetal and acetal formed from these reagents. The stoichiometry of each reaction is given in the problem.arrow_forwardWhat alcohols are formed from the reaction of ethylene oxide with the following organocuprates followed by the addition of acid?arrow_forwardWhen an aldehyde is treated with LiAlH4 followed by addition of H₂O, what general class of product results? ether secondary alcohol primary alcohol ketone tertiary alcoholarrow_forward
- Give the systematic (IUPAC) names for these molecules. Boononon cnolonongron. НаСНз CHОССH2СH2СНCHЗ CH3 phenyl propanoate |4-methyl pentane methanoat Incorrect. You mixed up the acyl and alkoxy portions of the molecule. Name the alkoxy part first, followed by the acyl part.arrow_forwardWhy can’t 2-methyl-2-propanol be prepared by the reduction of a carbonyl compound?arrow_forwardWhen trans-2-chloro-1-cyclohexanol is treated with a base, cyclohexene oxide is the product. However, when cis-2-chloro-1-cyclohexanol is treated with a base, the product is cyclohexanone. Why doesn’t the cis isomer yield the oxide?arrow_forward
- Describe how 1-ethylcyclohexanol can be prepared from cyclohexane. You can use any inorganic reagents, any solvents, and any organic reagents as long as they contain no more than two carbons.arrow_forwardWhat reactions and reagents can be used to make phenol from benzene if electrophilic aromatic substitution reactions are excluded and benzene is the only source of carbon?arrow_forwardThe following molecule belongs to a class of compounds called enediols; each carbon of the double bond carries an-OH group. Draw structural formulas for the a-hydroxyketone and the a-hydroxyaldehyde with which this enediol is in equilibrium. CH-OH a-Hydroxyaldehyde = C-OH= a -Hydroxyketone ČH3 An enediolarrow_forward
- Ethyl butyrate, CH3CH2CH2CO2CH2CH3, is an artificial fruit flavor commonly used in the food industry for such flavors as orange and pineapple. Its fragrance and taste are often associated with fresh orange juice, and thus it is most commonly used as orange flavoring.It can be produced by the reaction of butanoic acid with ethanol in the presence of an acid catalyst (H+): CH3CH2CH2CO2H(l)+CH2CH3OH(l)H+⟶CH3CH2CH2CO2CH2CH3(l)+H2O(l). The chemist discovers a more efficient catalyst that can produce ethyl butyrate with a 78.0% yield. How many grams would be produced from 8.50 gof butanoic acid and excess ethanol? Express your answer in grams to three significant figures.arrow_forwardEthyl butyrate, CH3CH2CH2CO2CH2CH3, is an artificial fruit flavor commonly used in the food industry for such flavors as orange and pineapple. Its fragrance and taste are often associated with fresh orange juice, and thus it is most commonly used as orange flavoring.It can be produced by the reaction of butanoic acid with ethanol in the presence of an acid catalyst (H+): CH3CH2CH2CO2H(l)+CH2CH3OH(l)H+⟶CH3CH2CH2CO2CH2CH3(l)+H2O(l) a) Given 7.70 g of butanoic acid and excess ethanol, how many grams of ethyl butyrate would be synthesized, assuming a complete 100% yield? b) A chemist ran the reaction and obtained 5.25 g of ethyl butyrate. What was the percent yield? c) The chemist discovers a more efficient catalyst that can produce ethyl butyrate with a 78.0% yield. How many grams would be produced from 7.70 g of butanoic acid and excess ethanol?arrow_forwardEthyl butyrate, CH3CH2CH2CO2CH2CH3, is an artificial fruit flavor commonly used in the food industry for such flavors as orange and pineapple. Its fragrance and taste are often associated with fresh orange juice, and thus it is most commonly used as orange flavoring.It can be produced by the reaction of butanoic acid with ethanol in the presence of an acid catalyst (H+): CH3CH2CH2CO2H(l)+CH2CH3OH(l)H+⟶CH3CH2CH2CO2CH2CH3(l)+H2O(l) Given 8.50 g of butanoic acid and excess ethanol, how many grams of ethyl butyrate would be synthesized, assuming a complete 100%yield? Express your answer in grams to three significant figures.arrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

