
Interpretation:
The percentages of neutral and protonated forms present in a solution of 0.0010M pyrimidine at pH = 7.3 are to be calculated if the pKa of pyrimidinium ion is 1.3.
Concept introduction:
pH And pKa are related by Henderson-Hasselbalch equation as
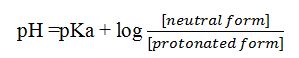
Knowing pH and pKa values, the ratio between the two forms and from which their percentages can be determined.
To calculate:
The percentages of neutral and protonated forms present in a solution of 0.0010M pyrimidine at pH = 7.3, if the pKa of pyrimidinium ion is 1.3.
Answer:
At pH = 7.3, almost 100% pyrimidine molecules exist in the neutral form.
Explanation:
Deprotonation of the ammonium ion of a base can be represented as,

The Henderson-Hasselbalch can be written as
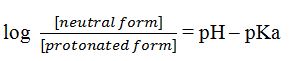
Substituting the values of pH and pKa, we get

Thus the concentration of neutral form is 106 times more than the protonated form. Hence almost 100% pyrimidine molecules exist in the neutral form.
Conclusion:
At pH = 7.3, almost 100% pyrimidine molecules exist in the neutral form.
Trending nowThis is a popular solution!

Chapter 24 Solutions
Organic Chemistry
- Would you expect an aqueous manganese (VII) oxide solution to have a pH greater or less than 7.0? Justify your answer.arrow_forwardCalculate the pH of the solution after the addition of each of the given amounts of 0.0584 M HNO3 to a 50.0 mL solution of 0.0750 M aziridine. The p?a of aziridinium is 8.04. What is the pH of the solution after the addition of 67.7 mL HNO3?arrow_forwardCalculate the pH of the solution after the addition of each of the given amounts of 0.0575 M HNO, to a 80.0 mL solution of 3. 0.0750 M aziridine. The pKa of aziridinium is 8.04. What is the pH of the solution after the addition of 0.00 mL HNO,? pH = What is the pH of the solution after the addition of 6.25 mL HNO, ? pH = What is the pH of the solution after the addition of a volume pH = of HNO, equal to half the equivalence point volume? What is the pH of the solution after the addition of 101 mL HNO,? pH = What is the pH of the solution after the addition of a volume of HNO, equal to the equivalence point? pH = What is the pH of the solution after the addition of 108 mL HNO,? pHarrow_forward
- The base ephedrine has a pKa value of 9.6. Calculate the theoretical end point pH when a 0.1 M solution of ephedrine is titrated with 0.1 M HCl.arrow_forwardCalculate the pH of the solution after the addition of each of the given amounts of 0.0554 M HNO3 to a 60.0 mL solution of 0.0750 M aziridine. The pka of aziridinium is 8.04. What is the pH of the solution after the addition of 0.00 mL HNO3? pH= What is the pH of the solution after the addition of 9.48 mL HNO3? pH= What is the pH of the solution after the addition of a volume of HNO3 equal to half the equivalence point volume? pH= What is the pH of the solution after the addition of 77.8 mL HNO3? pH= What is the pH of the solution after the addition of a volume of HNO3 equal to the equivalence point? pH= What is the pH of the solution after the addition of 85.7 mL HNO3? pH=arrow_forwardThe measured pH of a 0.100 M solution of triethylamine (NEt3) is 11.68. What pKa of triethylammonium (HNEt3+) is implied by this data? Enter your response to the nearest 0.01.arrow_forward
- Calculate the pH of the solution after the addition of each of the given amounts of 0.0603 M HNO3 to a 60.0 mL solution of 0.0750 M aziridine. The pKa of aziridinium is 8.04. What is the pH of the solution after the addition of 70.5 mL HNO3? pH= What is the pH of the solution after the addition of a volume of HNO3 equal to the equivalence point?pH= What is the pH of the solution after the addition of 77.9 mL HNO3? pH=arrow_forwardWhat molar ratio of HPO4 to H2PO4 in solution would produce a pH of 7.0? Phosphoric acid (H:PO4), a triprotic acid, has 3 pKa values: 2.14, 6.86, and 12.4. Hint: Only one of the pKa values is relevant here.arrow_forwardGiven that pKb for nitrite ion (NO-2) is 10.85, find the quotient [HNO2]/[NO-2] in a solution of sodium nitrite at (a) pH 2.00; (b) pH 10.00.arrow_forward
- Alizarin yellow R, Ka = 7.9 x 10-1 , is yellow in its protonated form (HX) and red in its ionized form (X). At what pH will alizarin yellow R be a perfect orange color?arrow_forwardThe dissociation of lactic acid to lactate is shown in the reaction. Lactic acid has a pk, of 3.86. + H* HO OH OH Lactic acid Lactate A solution containing a mixture of lactic acid and lactate was found to have a pH of 3.32. Calculate the ratio of the lactate concentration to the lactic acid concentration in this solution. [lactate] [lactic acid] 0.301 What fraction of the lactic acid/lactate mixture is lactic acid? fraction lactic acid =arrow_forwardWhat will be the pH of a buffer solution containing an acid with a pKa of 5.4 with an acid concentration equivalent to that of its conjugate base?arrow_forward
 Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning


