
(a)
Interpretation:
The products expected when D-mannose is reacted with
Concept introduction:
Tollens test is the chemical test for the identification of the presence of the aldehyde group in the compound.
Answer to Problem 24.34AP
The product obtained when D-mannose is reacted with
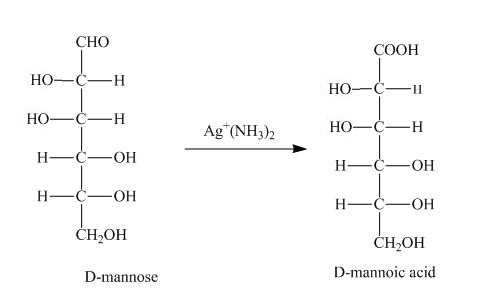
Explanation of Solution
The product obtained when D-mannose is reacted with

Figure 1
The Tollens reagent oxidizes the aldehyde group into carboxylic acid. The D-mannose is oxidized by the
The product obtained when D-mannose is reacted with
(b)
Interpretation:
The products expected when D-mannose is reacted with dilute
Concept introduction:
There are two forms of sugars that is
Answer to Problem 24.34AP
The product obtained when D-mannose is reacted with dilute
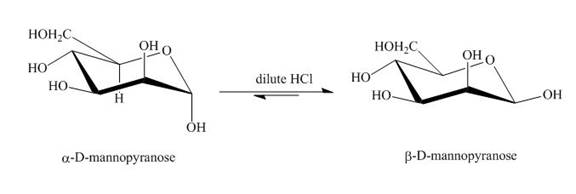
Explanation of Solution
The product obtained when D-mannose is reacted with dilute
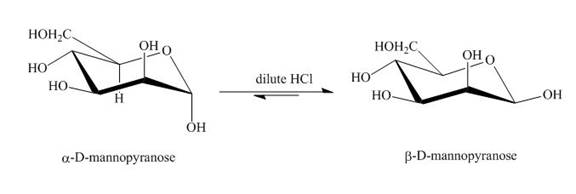
Figure 2
The conversion of both forms of sugar into each other in the presence of acid and base. That is the alpha form is converted beta and vice-versa. This change occurs until an equilibrium mixture of both compounds is obtained.
The product obtained when D-mannose is reacted with dilute
(c)
Interpretation:
The products expected when D-mannose is reacted with dilute
Concept introduction:
There are two forms of sugars that is
Answer to Problem 24.34AP
The product obtained when D-mannose is reacted with dilute
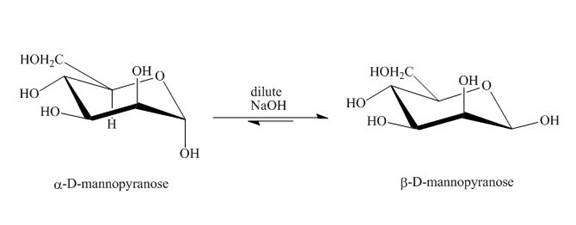
Explanation of Solution
The product obtained when D-mannose is reacted with dilute

Figure 3
The conversion of both forms of sugar into each other in the presence of acid or base. That is the alpha form is converted beta and vice-versa. This change occurs until an equilibrium mixture of both compounds is obtained.
The product obtained when D-mannose is reacted with dilute
(d)
Interpretation:
The products expected when D-mannose is reacted with
Concept introduction:
The aldehyde group on oxidation changes into a carboxylic acid. The oxidation of the aldehyde group present in the aldoses is done by the bromine and water into carboxylic acids. The carboxylic acid of the aldoses thus formed is converted into lactones form.
Answer to Problem 24.34AP
The product obtained when D-mannose is reacted with
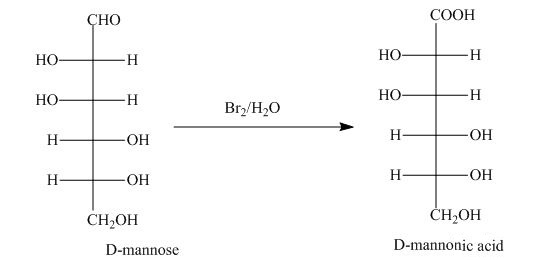
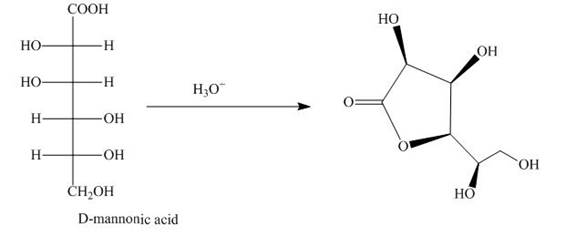
Explanation of Solution
The product obtained when D-mannose is reacted with


Figure 4
Bromine and water oxidize the aldoses into carboxylic acids. The carboxylic acid thus formed is found in the form of lactones. Lactones formed are more stable in the five-membered
The product obtained when D-mannose is reacted with
(e)
Interpretation:
The products expected when D-mannose is reacted with
Concept introduction:
A monosaccharide is converted into cyclic acetals on reaction with alcohols in the presence of acidic conditions. The hydroxide group right to the oxygen atom in the pyranose ring structure is methylated and result in the formation of acetal.
Answer to Problem 24.34AP
The product obtained when D-mannose is reacted with
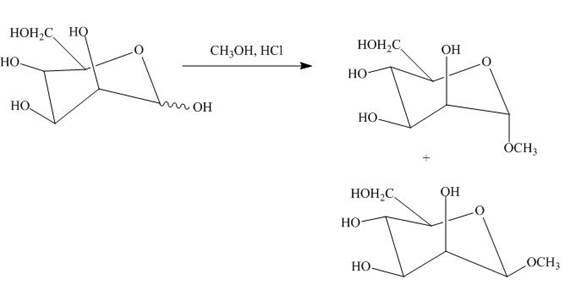
Explanation of Solution
The product obtained when D-mannose is reacted with

Figure 5
The D-mannose on reaction with methanol and hydrochloric acid is converted into the acetal. The acetal formed is found in both forms alpha and beta regardless of the configuration of D-mannose.
The product obtained when D-mannose is reacted with
(f)
Interpretation:
The products expected when D-mannose is reacted with acetic anhydride/pyridine is to be stated.
Concept introduction:
Esterification reaction of alcohol units of monosaccharide is also done. The esterification of the alcohol units is done in the presence of an excess of acetic anhydride and pyridine. All the alcohol units present are acylated.
Answer to Problem 24.34AP
The product obtained when D-mannose is reacted with acetic anhydride/pyridine is shown below.
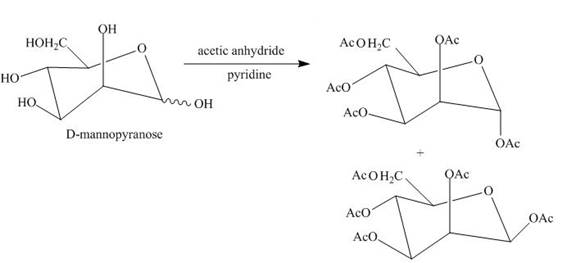
Explanation of Solution
The product obtained when D-mannose is reacted with acetic anhydride/pyridine is shown below.

Figure 6
Esterification of all the alcohols units present in the D-mannose is done in the presence of acetic anhydride and pyridine.
The product obtained when D-mannose is reacted with acetic anhydride/pyridine is shown in Figure 6.
(g)
Interpretation:
The product obtained when the product of part (d) is reacted with
Concept introduction:
Ruff’s degradation is the degradation of the sugar molecule by one carbon unit. The aldose is oxidized to the carboxylic acids using bromine water. The carboxylic acid is converted into calcium salt using the calcium hydroxide base which on further reaction with
Answer to Problem 24.34AP
The product obtained when the product of part (d) is reacted with
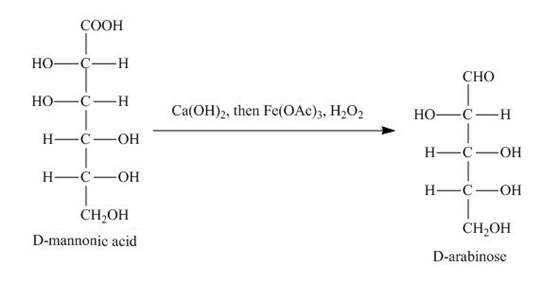
Explanation of Solution
The product of part (d) is shown below.
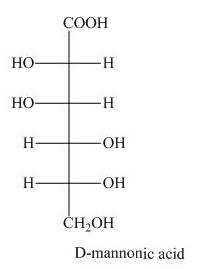
Figure 7
The product obtained when the product of part (d) is reacted with

Figure 8
The product obtained is the Ruff’s degradation product of the D-mannose. The D-mannonic acid is converted into calcium salt in the first step and then on reaction with
The product obtained when the product of part (d) is reacted with
(h)
Interpretation:
The product obtained when the product of part (e) is reacted with
Concept introduction:
The alkylation of the hydroxyl group of sugars is an important reaction. The alkylation of hydroxyl groups is done with the help of alkylating agent
Answer to Problem 24.34AP
The product obtained when the product of part (e) is reacted with
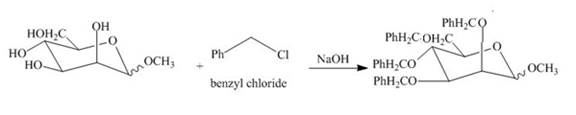
Explanation of Solution
The product of part (e) is shown below.
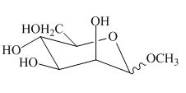
Figure 9
The product obtained when the product of part (e) is reacted with

Figure 10
The methyl D-mannopyranoside is alkylated in the strong base sodium hydroxide. The sodium hydroxide takes up the acidic proton of alcohol groups and converts them to alkoxide ion form. This alkoxide ion then substitutes the chloride group in the
The product obtained when the product of part (e) is reacted with
Want to see more full solutions like this?
Chapter 24 Solutions
Organic Chemistry
- Predict the products obtained from the reaction of triolein with the following reagents.(a) NaOH in water (b) H2 and a nickel catalyst (c) Br2 in CCl4arrow_forwardPredict the products obtained from the reaction of triolein with the following reagents.(a) NaOH in water (b) H2 and a nickel catalyst (c) Br2 in CCl4(d) ozone, then dimethyl sulfide (e) warm KMnO4 in water (f) CH2I2>Zn(Cu)arrow_forwardPredict the products formed when cyclohexanecarbaldehyde reacts with the following reagents.(a) PhMgBr, then H3O+ (b) Tollens reagent (c) semicarbazide and weak acid(d) excess ethanol and acid (e) propane-1,3-diol, H+ (f) zinc amalgam and dilute hydrochloric acidarrow_forward
- 1. Draw structures corresponding to the following IUPAC names: (a) 4-Methylpentanoic acid (b) o-Hydroxybenzoic acid (c) 2,2-Dimethylpropanoyl chloride (d) trans-2-Methylcyclohexanecarboxamide (e) p-Methylbenzoic anhydride (f) p-Bromobenzonitrilearrow_forwardDraw structures corresponding to the following names: (a) 3-hydroxyhexanoic acid (b) 2-iodo-2-methyloctanoic acid (c) 2-butynoic acid (d) 5-ethyl-6-oxoheptanoic acid (e) o-Hydroxybenzoic acid (f) cis-3-isopropylcyclohexanecarboxylic acidarrow_forwardDraw the structural formulas of the following compounds:(a) 2,4-Dinitroacetophenone(b) 2,4-Dihydroxycyclopentanone(c) 2-Methoxy-2-methylpropane(d) 2,3,4-Trimethylpentan-3-olarrow_forward
- (a) Predict the product of the reaction of KOH with 1-amino propane. (b) Predict the product of a deprotonated ethanol (an “ethanolate anion", O-CH2-CH3) with phenol (hydroxybenzene). (c) Predict the product of propanoic acid with deprotonated ethanol (an “ethanolate anion", O-CH2-CH3).arrow_forwardPredict the products obtained from the reaction of triolein with the following reagents.(a) NaOH in water (b) H2 and a nickel catalystarrow_forward(b) 3-methyl-2-butanol reacts with concentrated sulphuric acid to form 2-methyl-2- butene. Write the mechanism for the reaction.arrow_forward
- (a) Draw the structure of the following :(i) p-Methylbenzaldehyde (ii) 4-Methylpent-3-en-2-one(b) Give chemical tests to distinguish between the following pairs of compounds :(i) Benzoic acid and Ethyl benzoate, (ii) Benzaldehyde and Acetophenone.(iii) Phenol and Benzoic acid.arrow_forwardPredict the major products formed when benzoyl chloride (PhCOCl) reacts with the following reagents.(a) ethanol (b) sodium acetate (c) anilinearrow_forwardCompound X (C4H9Br) reacts by heating with NaOH in H2O to form Y. The compound Y then undergoes acid catalysed hydration by H2SO4 in 180°C to form 2-methyl prop-1-ene. (e) Determine the structure of X and Y. (f) Predict a MAJOR product when compound Y reacts with H2SO4 in 140°C. (g) Draw a structural isomer of X. Name the isomer using IUPAC nomenclature. (h) Describe a chemical test to distinguish between compound Y and 1-butanol.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





