
Concept explainers
(a)
Interpretation:
The Fischer projection, Haworth projection, and a line-and wedge structure for
Concept introduction:
Fischer projection is the two dimensional structure of a three dimensional compound. In it, the substituents of an asymmetric center are shown on horizontal and vertical lines. Haworth projection is the condensed cyclic representation of glucose, consisting of a pyranose ring.
Answer to Problem 24.7P
The Fischer projection, Haworth projection, and a line-and wedge structure for
Explanation of Solution
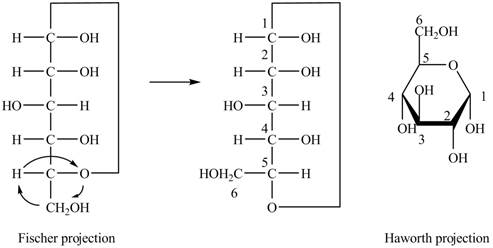
The Fischer projection of
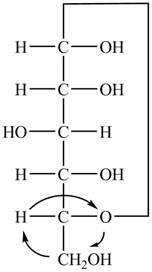
Figure 1
The Fischer projection of
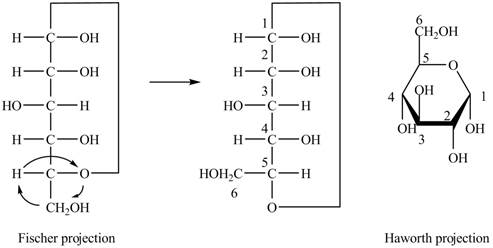
Figure 2
The chair conformation of
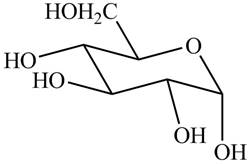
Figure 3
The Fischer projection, Haworth projection, and a line-and wedge structure for
(b)
Interpretation:
The Fischer projection, Haworth projection, and a line-and wedge structure for
Concept introduction:
Fischer projection is the two dimensional structure of a three dimensional compound. In it, the substituents of an asymmetric center are shown on horizontal and vertical lines. Haworth projection is the condensed cyclic representation of glucose, consisting of a pyranose ring.
Answer to Problem 24.7P
The Fischer projection, Haworth projection, and a line-and wedge structure for
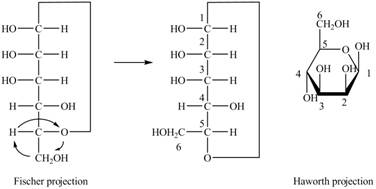
Explanation of Solution
The Fischer projection of
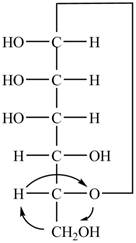
Figure 4
The Fischer projection of

Figure 5
The chair conformation of
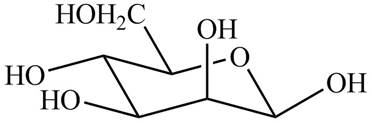
Figure 6
The Fischer projection, Haworth projection, and a line-and wedge structure for
(c)
Interpretation:
The Fischer projection, Haworth projection, and a line-and wedge structure for
Concept introduction:
Fischer projection is the two dimensional structure of a three dimensional compound. In it, the substituents of an asymmetric center are shown on horizontal and vertical lines. Haworth projection is the condensed cyclic representation of glucose, consisting of a pyranose ring.
Answer to Problem 24.7P
The Fischer projection, Haworth projection, and a line-and wedge structure for
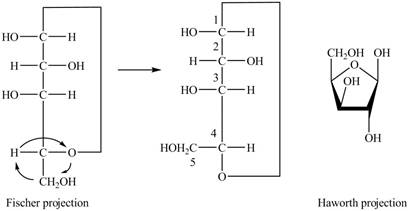
Explanation of Solution
The Fischer projection of
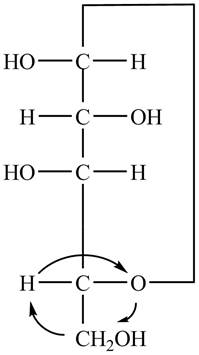
Figure 7
The Fischer projection of
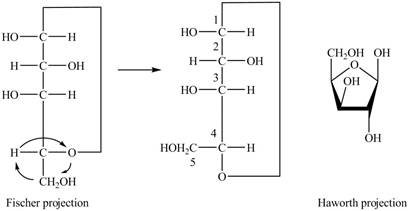
Figure 8
The chair conformation of
The Fischer projection, Haworth projection, and a line-and wedge structure for
(d)
Interpretation:
The Fischer projection, Haworth projection, and a line-and wedge structure for
Concept introduction:
Fischer projection is the two dimensional structure of a three dimensional compound. In it, the substituents of an asymmetric center are shown on horizontal and vertical lines. Haworth projection is the condensed cyclic representation of glucose, consisting of a pyranose ring.
Answer to Problem 24.7P
The Fischer projection, Haworth projection, and a line-and wedge structure for
Explanation of Solution
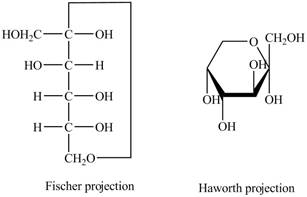
The Fischer projection of
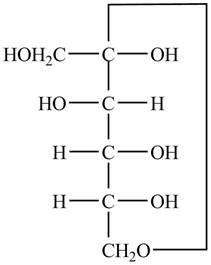
Figure 9
The Fischer projection of

Figure 10
The chair conformation of
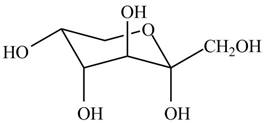
Figure 11
The Fischer projection, Haworth projection, and a line-and wedge structure for
(e)
Interpretation:
The Fischer projection, Haworth projection, and a line-and wedge structure for
Concept introduction:
Fischer projection is the two dimensional structure of a three dimensional compound. In it, the substituents of an asymmetric center are shown on horizontal and vertical lines. Haworth projection is the condensed cyclic representation of glucose, consisting of a pyranose ring.
Answer to Problem 24.7P
The Fischer projection, Haworth projection, and a line-and wedge structure for
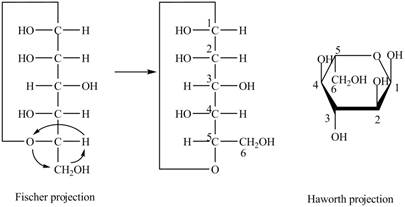
Explanation of Solution
The Fischer projection of
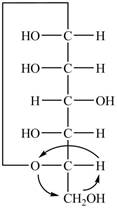
Figure 12
The Fischer projection of

Figure 13
The chair conformation of
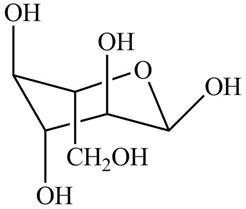
Figure 14
The Fischer projection, Haworth projection, and a line-and wedge structure for
(f)
Interpretation:
The Fischer projection, Haworth projection, and a line-and wedge structure for a mixture of the
Concept introduction:
Fischer projection is the two dimensional structure of a three dimensional compound. In it, the substituents of an asymmetric center are shown on horizontal and vertical lines. Haworth projection is the condensed cyclic representation of glucose, consisting of a pyranose ring.
Answer to Problem 24.7P
The Fischer projection, Haworth projection, and a line-and wedge structure for a mixture of the
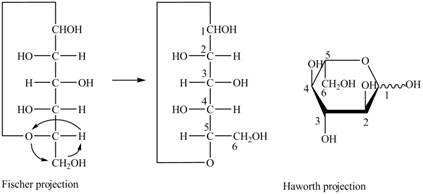
Explanation of Solution
The Fischer projection for a mixture of the
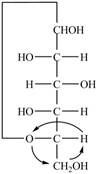
Figure 15
The Fischer projection for a mixture of the

Figure 16
The chair conformation for a mixture of the
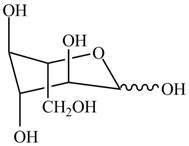
Figure 17
The Fischer projection, Haworth projection, and a line-and wedge structure for
Want to see more full solutions like this?
Chapter 24 Solutions
Organic Chemistry
- Draw Haworth projections for each of the following molecules: (a) α-d-allopyranose, (b) β-d-allopyranose, (c) α-d-allofuranose, (d) β-d-allofuranose.arrow_forward(a) Which of the d-aldopentoses will give optically active aldaric acids on oxidation with HNO3 ?(b) Which of the d-aldotetroses will give optically active aldaric acids on oxidation with HNO3 ?(c) Sugar X is known to be a d-aldohexose. On oxidation with HNO3, X gives an optically inactive aldaric acid. WhenX is degraded to an aldopentose, oxidation of the aldopentose gives an optically active aldaric acid. Determine thestructure of X.(d) Even though sugar X gives an optically inactive aldaric acid, the pentose formed by degradation gives an opticallyactive aldaric acid. Does this finding contradict the principle that optically inactive reagents cannot form opticallyactive products?(e) Show what product results if the aldopentose formed from degradation of X is further degraded to an aldotetrose.Does HNO3 oxidize this aldotetrose to an optically active aldaric acid?arrow_forwardQuinapril (trade name Accupril) is used to treat high blood pressure and congestive heart failure. One step in the synthesis of quinapril involves reaction of the racemic alkyl bromide A with a single enantiomer of the amino ester B. (a) What two products are formed in this reaction? (b) Given the structure of quinapril, which one of these two products is needed tosynthesize the drug?arrow_forward
- Draw the structural formulas of the following compounds:(a) 2,4-Dinitroacetophenone(b) 2,4-Dihydroxycyclopentanone(c) 2-Methoxy-2-methylpropane(d) 2,3,4-Trimethylpentan-3-olarrow_forward(a) Explain how NaBH4 in CH3OH can reduce hemiacetal A to butane-1,4-diol (HOCH2CH2CH2CH2OH). (b) What product is formed when A is treated with Ph3P = CHCH2CH(CH3)2? (c) The drug isotretinoin is formed by reaction of X and Y. What is the structure of isotretinoin? Although isotretinoin (trade name Accutane or Roaccutane) is used for the treatment of severe acne, it is dispensed under strict controls because it also causes birth defects.arrow_forwardQuinapril (trade name Accupril) is used to treat high blood pressure and congestive heart failure. One step in the synthesis of quinapril involves reaction of the racemic alkyl bromide A with a single enantiomer of the amino ester B. (a) What two products are formed in this reaction? (b) Given the structure of quinapril, which one of these two products is needed to synthesize the drug?arrow_forward
- Coibacin B (shown below) is a natural product that exhibits potent anti-inflammatory activity and potential activity in the treatment of leishmaniasis, a disease caused by certain parasites (Org. Lett. 2012, 14, 3878-3881): (a) Assign the configuration (R or S) of each chirality center (labeled A to C) in coibacin B. (b) Identify the number of possible stereoisomers for this compound, assuming that the geometry of the alkenes are fixed. Choices are given below and write the CAPITAL LETTER of your choice. A. 2 В. 4 С. 8 D. 16 ANSWERS: (a) A. В. C. (b)arrow_forwardA key step in the synthesis of the narcotic analgesic meperidine (trade name Demerol) is the conversion of phenylacetonitrile to X. (a) What is the structure of X? (b) What reactions convert X to meperidine?arrow_forwardDraw all possible constitutional isomers of a triacylglycerol formed from one mole each of palmitic, oleic, and linoleic acids. Locate the tetrahedral stereogenic centers in each constitutional isomer.arrow_forward
- 5:51 Draw examples of the following: (a) A meso compound with the formula C8H18 (b) A meso compound with the formula C9H20 (c) A compound with two chirality centers, one R and the other Sarrow_forwardOne step in the gluconeogenesis pathway for the biosynthesis of glucose is the partial reduction of 3-phosphoglycerate to give glyceraldehyde 3-phosphate. The process occurs by phosphorylation with ATP to give 1,3-bisphosphoglycerate, reaction with a thiol group on the enzyme to give an enzyme-bound thioester, and reduction with NADH. -OPO3²- Enz-SH H-C-OH ATP CH₂OPO3²- 3-phosphoglycerate O 0-0--0 O ADP CH₂CH3 substitute for 1,3-bisphosphoglycerate C H-C-OH CH₂OPO3²- 1,3-bisphosphoglycerate O=C CH3-SH substitute for Enz-SH H H-C-OH | CH₂OPO3²- PO4³- O. S-Enz H-C-OH glyceraldehyde 3-phosphate Propose a structure for the first intermediates in the reaction of 1,3-bisphosphoglycerate with a thiol group on the enzyme to form an enzyme-bound thioester. Assume a basic group on the enzyme catalyzes the formation of this intermediate. To simplify the drawing process, substitute the structures below for the 1,3-bisphosphoglycerate and Enz-SH. CH₂OPO3²- (Enzyme-bound thioester) NADH/H* NAD*,…arrow_forward31. Which of the following statements about cholesterol is not correct? CH. HO Cholesterol 16 (a) Cholesterol is a steroid that contains a tetracyclic ring system. (b) Cholesterol is a steroid that contains 8 chiral carbons and can form 28 or 256 stereoisomers. (c) Each atom or group attached to a ring-junction carbon (i.e., carbons a-e) is in a trans or axial position. Because of this the tetracyclic ring system is mostly flat. (d) Cholesterol is used to synthesized vitamin D, bile acids, sex hormones, and adrenocorticoid hormones. (e) Cholesterol is not found in the cell membranes of animals.arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning

