
Concept explainers
a)
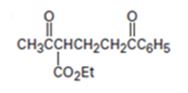
Interpretation:
The nucleophilic donor and electrophilic acceptor that react in a Michael reaction to yield the compound shown are to be given.
Concept introduction:
Michael reaction involves the conjugate addition of a stable enolate ion derived from a β-ketoesters or β-diketones or β-ketonitriles or malonic esters (donors) to an unhindered α,β-unsaturated
To give:
The nucleophilic donor and electrophilic acceptor that react in a Michael reaction to yield the compound shown.
Answer to Problem 62AP
The nucleophilic donor and electrophilic acceptor that react in a Michael reaction to yield the compound shown are
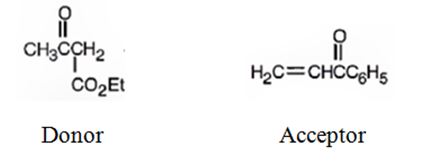
Explanation of Solution
An analysis of the structure of the compound indicates that it is formed by the reaction between the ethylacetoacetate (nucleophilic donor) and phenyl vinyl ketone (electrophilic acceptor).
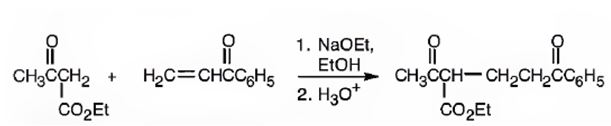
The nucleophilic donor and electrophilic acceptor that react in a Michael reaction to yield the compound shown are
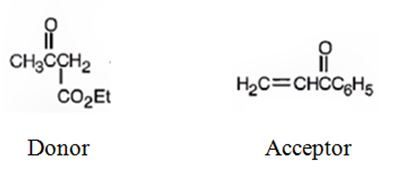
b)
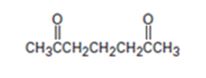
Interpretation:
The nucleophilic donor and electrophilic acceptor that react in a Michael reaction to yield the compound shown are to be given.
Concept introduction:
Michael reaction involves the conjugate addition of a stable enolate ion derived from a β-ketoesters or β-diketones or β-ketonitriles or malonic esters (donors) to an unhindered α,β-unsaturated ketones or aldehydes or esters or thioesters or nitriles or amides or nitro compounds (acceptors). The enolate ion from the donor attacks the double bond in acceptor. A new bond is formed between the α-carbon of the donor and the β-carbon of the unsaturated ester.
To give:
The nucleophilic donor and electrophilic acceptor that react in a Michael reaction to yield the compound shown.
Answer to Problem 62AP
The nucleophilic donor and electrophilic acceptor that react in a Michael reaction to yield the compound shown are

Explanation of Solution
An analysis of the structure of the compound indicates that it is formed by the reaction between the ethylacetoacetate (nucleophilic donor) and methyl vinyl ketone (electrophilic acceptor).
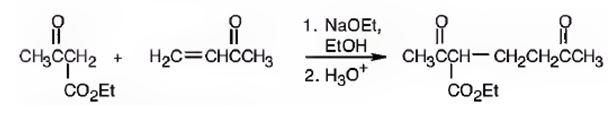
The nucleophilic donor and electrophilic acceptor that react in a Michael reaction to yield the compound shown are

c)
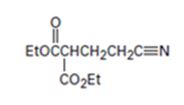
Interpretation:
The nucleophilic donor and electrophilic acceptor that react in a Michael reaction to yield the compound shown are to be given.
Concept introduction:
Michael reaction involves the conjugate addition of a stable enolate ion derived from a β-ketoesters or β-diketones or β-ketonitriles or malonic esters (donors) to an unhindered α,β-unsaturated ketones or aldehydes or esters or thioesters or nitriles or amides or nitro compounds (acceptors). The enolate ion from the donor attacks the double bond in acceptor. A new bond is formed between the α-carbon of the donor and the β-carbon of the unsaturated ester.
To give:
The nucleophilic donor and electrophilic acceptor that react in a Michael reaction to yield the compound shown.
Answer to Problem 62AP
The nucleophilic donor and electrophilic acceptor that react in a Michael reaction to yield the compound shown are

Explanation of Solution
An analysis of the structure of the compound indicates that it is formed by the reaction between the ethylacetoacetate (nucleophilic donor) and vinyl nitrile (electrophilic acceptor).
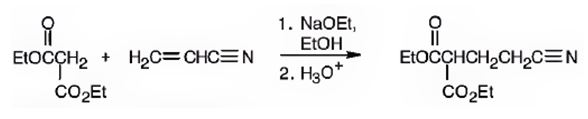
The nucleophilic donor and electrophilic acceptor that react in a Michael reaction to yield the compound shown are

d)
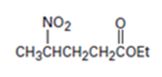
Interpretation:
The nucleophilic donor and electrophilic acceptor that react in a Michael reaction to yield the compound shown are to be given.
Concept introduction:
Michael reaction involves the conjugate addition of a stable enolate ion derived from a β-ketoesters or β-diketones or β-ketonitriles or malonic esters (donors) to an unhindered α,β-unsaturated ketones or aldehydes or esters or thioesters or nitriles or amides or nitro compounds (acceptors). The enolate ion from the donor attacks the double bond in acceptor. A new bond is formed between the α-carbon of the donor and the β-carbon of the unsaturated ester.
To give:
The nucleophilic donor and electrophilic acceptor that react in a Michael reaction to yield the compound shown.
Answer to Problem 62AP
The nucleophilic donor and electrophilic acceptor that react in a Michael reaction to yield the compound shown are

Explanation of Solution
An analysis of the structure of the compound indicates that it is formed by the reaction between nitro ethane (nucleophilic donor) and ethylacrylate (electrophilic acceptor).
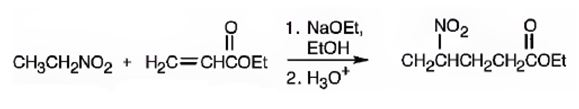
The nucleophilic donor and electrophilic acceptor that react in a Michael reaction to yield the compound shown are
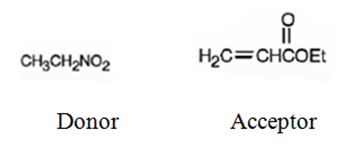
e)
Interpretation:
The nucleophilic donor and electrophilic acceptor that react in a Michael reaction to yield the compound are to be given.
Concept introduction:
Michael reaction involves the conjugate addition of a stable enolate ion derived from a β-ketoesters or β-diketones or β-ketonitriles or malonic esters (donors) to an unhindered α,β-unsaturated ketones or aldehydes or esters or thioesters or nitriles or amides or nitro compounds (acceptors). The enolate ion from the donor attacks the double bond in acceptor. A new bond is formed between the α-carbon of the donor and the β-carbon of the unsaturated ester.
To give:
The nucleophilic donor and electrophilic acceptor that react in a Michael reaction to yield the compound shown.
Answer to Problem 62AP
The nucleophilic donor and electrophilic acceptor that react in a Michael reaction to yield the compound shown are

Explanation of Solution
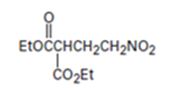
An analysis of the structure of the compound indicates that it is formed by the reaction between the ethylsuccinate (nucleophilic donor) and nitro ethene (electrophilic acceptor).
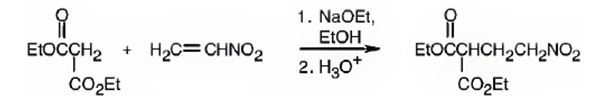
The nucleophilic donor and electrophilic acceptor that react in a Michael reaction to yield the compound shown are

f)
Interpretation:
The nucleophilic donor and electrophilic acceptor that react in a Michael reaction to yield the compound shown are to be given.
Concept introduction:
Michael reaction involves the conjugate addition of a stable enolate ion derived from a β-ketoesters or β-diketones or β-ketonitriles or malonic esters (donors) to an unhindered α,β-unsaturated ketones or aldehydes or esters or thioesters or nitriles or amides or nitro compounds (acceptors). The enolate ion from the donor attacks the double bond in acceptor. A new bond is formed between the α-carbon of the donor and the β-carbon of the unsaturated ester.
To give:
The nucleophilic donor and electrophilic acceptor that react in a Michael reaction to yield the compound shown.
Answer to Problem 62AP
The nucleophilic donor and electrophilic acceptor that react in a Michael reaction to yield the compound shown are
Explanation of Solution
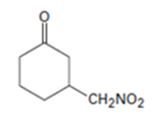
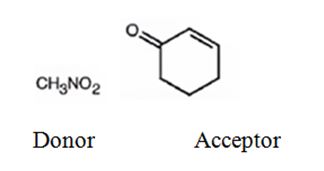
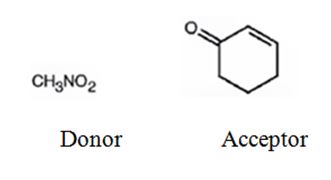
An analysis of the structure of the compound indicates that it is formed by the reaction between nitro methane (nucleophilic donor) and 2-cyclopentenone (electrophilic acceptor).
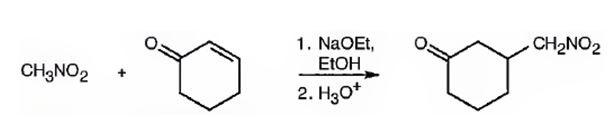
The nucleophilic donor and electrophilic acceptor that react in a Michael reaction to yield the compound shown are
Want to see more full solutions like this?
Chapter 23 Solutions
Organic Chemistry
- Choose the best reagents to complete the following reactions.arrow_forwardWrite out the synthesis for each of the following reactions. Show the product and reagents for each step in each synthesis.?arrow_forwardWhat explains why many aldehydes and ketones can undergo self- condensation reactions in basic conditions? The alpha carbon can lose a proton and act like a nucleophile and the carbonyl carbon a an electrophile O The alpha carbon can gain a proton and act like an electrophile and the carbonyl carbon is a nucleophile O The oxygen of the carbonyl group can attack the carbon of the carbonyl group Only esters can undergo self-condensation reactionsarrow_forward
- Which reagent(s), if any, may be used to carry out the following reactionarrow_forwardWhat is the source of the nucleophilic attack? How does the oxygen get protonated? What causes the elimination reaction?arrow_forward2 Moin H3C H H₂C C⇒x= base :0: OH Hori H3C H. The aldol reaction is a carbonyl condensation reaction between two carbonyl partners and involves a combination of nucleophilic addition and a-substitution steps. One partner is converted into an enolate ion nucleophile and adds to the electrophilic carbonyl group of the second partner. In the classic aldol reaction, the carbonyl partners are aldehydes or ketones, although aldehydes are more reactive. The product is a B-hydroxy carbonyl compound. Under reaction conditions slightly more vigorous than those employed for the aldol reaction, the ß-hydroxyl group is eliminated in an E1cB dehydration to give an a,ß-unsaturated carbonyl compound. Draw curved arrows to show the movement of electrons in this step of the mechanism. Arrow-pushing Instructions H₂C Ἡ :0: heat H Home H3C + H₂Oarrow_forward
- Complete the following reactions with the appropriate reagents or products. 1 mol HBr H₂C 2 H₂ Pd 2 mol HCI ECH ? H₂C BH₂ H₂O₂, H₂O -CH ₂arrow_forwardPleas explain how this process occurs. Identify SN1, SN2, E2, E1, nucleophiles and electrophiles.arrow_forwardSuccinic acid can be synthesized by the following series of reactions from acetylene. Show the reagents and experimental conditions necessary to carry out this synthesis. НО OH НО. HO H- H. HO, ОН Acetylene 2-Butyne-1,4-diol 1,4-Butanediol Butanedioic acid (Succinic acid)arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





