
Concept explainers
(a)
Interpretation:
The Lewis structure of
Concept Introduction:
Valence Shell Electron Pair Repulsion model predicts shape by inclusion of bond angles and most distant arrangement of atoms that leads to minimum repulsion.
For molecules that have lone pairs around central atom, lone pairs influence shape, because there are no atoms at the positions occupied by these lone pairs. The key rule that governs the molecular shape, in this case, is the extent of lone pair–lone pair repulsions are far greater than lone bond pair or bond pair-bond pair repulsions. The table that summarized the molecular shapes possible for various combinations of bonded and lone pairs are given as follows:
(a)
Answer to Problem 2E.20E
The shape for
Explanation of Solution
Total valence electrons are sum of the valence electrons on each atom in
The skeleton structure in
These 15 electron pairs are assigned as lone pairs of each of the
Hence, the Lewis structure
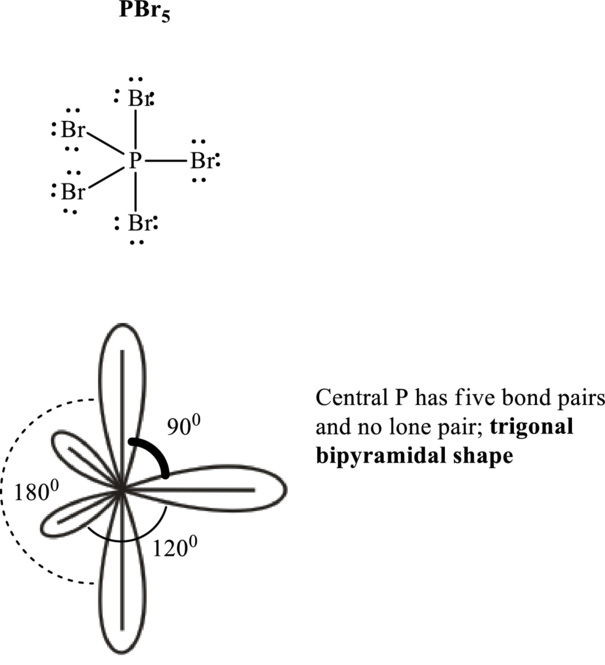
If lone pairs are represented by E, central atom with A and other attached bond pairs by X, then for any trigonal pyramidal geometry the VSEPR formula is predicted as
It is evident that in
The bond angles are
(b)
Interpretation:
The Lewis structure of
Concept Introduction:
Refer to part (a).
(b)
Answer to Problem 2E.20E
The shape for
Explanation of Solution
Total valence electrons are sum of the valence electrons on each atom in
The skeleton structure in
These 11 electron pairs are allotted as lone pairs of each of the fluorine, oxygen atoms and central xenon to satisfy respective octets. Thus, the Lewis structure and corresponding VSEPR geometry
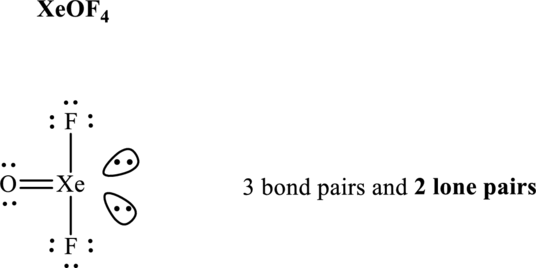
It is evident that in
(c)
Interpretation:
The Lewis structure of
Concept Introduction:
Refer to part (a).
(c)
Answer to Problem 2E.20E
The shape for
Explanation of Solution
Total valence electrons are sum of the valence electrons on each chlorine and central iodine in
The skeleton structure in
These 15 electron pairs are allotted as lone pairs to each of the
It is evident that in
(d)
Interpretation:
The Lewis structure of
Concept Introduction:
Refer to part (a).
(d)
Answer to Problem 2E.20E
The shape for
Explanation of Solution
Total valence electrons are sum of the valence electrons on each
The skeleton structure in
These 11 electron pairs are allotted as lone pairs of each of the fluorine atoms and central iodine to satisfy respective octets. Hence, the Lewis structure and corresponding VSPER geometry in
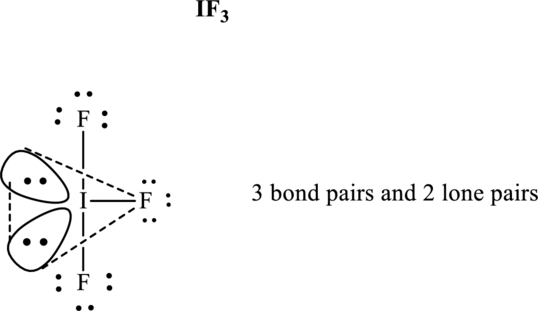
It is evident that in
Lone pairs tend to occupy the equatorial locations of trigonal plane so that they are
(e)
Interpretation:
The Lewis structure of
Concept Introduction:
Refer to part (a).
(e)
Answer to Problem 2E.20E
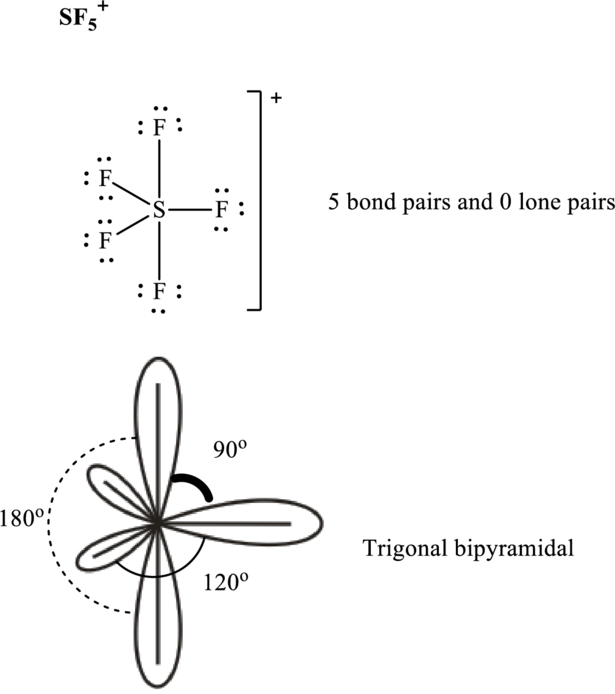
The shape for
Explanation of Solution
Total valence electrons are sum of the valence electrons on each atom in
The skeleton structure in
These 10 electron pairs are allotted as lone pairs or multiple bonds to satisfy respective octets. Hence, the Lewis structure and corresponding VSPER geometry in
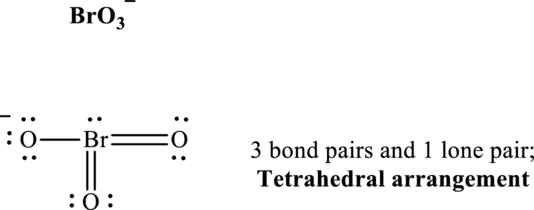
It is evident that in
If lone pairs are represented by E, central atom with A and other attached bond pairs by X, then for any see-saw species the VSEPR formula is predicted as
The bond pairs in
Want to see more full solutions like this?
Chapter 2 Solutions
Chemical Principles: The Quest for Insight
- Using the bond dissociation enthalpies in Table 8.8, estimate the enthalpy of combustion of gaseous methane, CH4, to give water vapor and carbon dioxide gas.arrow_forwardFormamide, HC(O)NH2, is prepared at high pressures from carbon monoxide and ammonia, and serves as an industrial solvent (the parentheses around the O indicate that it is bonded only to the carbon atom and that the carbon atom is also bonded to the H and the N atoms). Two resonance forms (one with formal charges) can be written for formamide. Write both resonance structures, and predict the bond angles about the carbon and nitrogen atoms for each resonance form. Are they the same? Describe how the experimental determination of the HNH bond angle could be used to indicate which resonance form is more important.arrow_forwardBest Lewis Formula and Molecular Geometry A student writes the Lewis electron-dot formula for the carbonate anion, CO32, as a Does this Lewis formula obey the octet rule? Explain. What are the formal charges on the atoms? Try describing the bonding for this formula in valence bond terms. Do you have any difficulty doing this? b Does this Lewis formula give a reasonable description of the electron structure, or is there a better one? If there is a better Lewis formula, write it down and explain why it is better. c The same student writes the following resonance description for CO2: Is there something wrong with this description? (What would you predict as the geometries of these formulas?) d Is one or the other formula a better description? Could a value for the dipole moment help you decide? e Can you write a Lewis formula that gives an even better description of CO2? Explain your answer.arrow_forward
- Write the Lewis structures for the following species, and indicate whether each is an odd-electron species, an electron-deficient species, or an expanded valence shell species. (a) BI3 (b) IF5 (c) HN2arrow_forwardChemical species are said to be isoelectronic if they have the same Lewis structure (regardless of charge). Consider these ions and write a Lewis structure for a neutral molecule that is isoelectronic with them. (a) CN–, (b) NH4+ (c) CO3 2–arrow_forwardKeeping in mind that some elements violate the octet rule, draw a Lewis structure for each compound: (a) BeH 2; (b) PCl 5.arrow_forward
- a) Methane 1CH42 and the perchlorate ion 1ClO4- 2 are both described as tetrahedral. What does this indicate about their bond angles? (b) The NH3 molecule is trigonal pyramidal, while BF3 is trigonal planar. Which of these molecules is flat?arrow_forwardWrite Lewis structures for the following: (c) C2F6 (contains a C¬C bond), (d) AsO3 3 -, (e) H2SO3 (H is bonded to O), (f) NH2Cl.. Arrange the bonds in each of the following sets in order of increasing polarity: (a) C¬F, O¬F, Be¬F; (b) O¬Cl, S¬Br, C¬P; (c) C¬S, B¬F, N¬O. What is the Lewis symbol for each of the following atoms or ions? (a) K, (b) As, (c) Sn2 + , (d) N3 Write electron configurations for the following ions and determine which have noble-gas configurations: (a) Cd2+, (b) P3-, (c) Zr4+arrow_forward. Assume that the third-period element phosphorus forms a diatomic molecule, P2, in an analogous way as nitrogen does to form N2. (a) Write the electronic configuration for P2. Use [Ne2] to represent the electron configuration for the first two periods. (b) Calculate its bond order. (c) What are its magnetic properties (diamagnetic or paramagnetic)?arrow_forward
- 5. (i) Provide possible resonance structure, (ii) evaluate the resonance structures with formal charge to identify the best structure, (iii) provide the resonance hybrid, the (iv) molecular geometry, and (v) discuss the bond angles. ClO, PO, 3-arrow_forwardThe structural formulas for ethanol, CH3CH2OH, and propene, CH;CH=CH,2, are нн H Н—С—С—0—н H-C-C=C-H нн H H H Ethanol Propene (a) Complete the Lewis structure for each molecule showing all valence electrons. (b) Using the VSEPR model, predict all bond angles in each molecule.arrow_forward1. Draw the Lewis structures for each of the following ions or molecules. For each, give (i) the molecular shape, (ii) the electron pair geometry at the central atom, and (iii) the hybridization of the central atom. (a) POF3 (b) XeO₂F3+ (c) BrCl₂ (d) N3 (the central atom is N; two other N's are bonded to it) (e) PF3arrow_forward
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning




