
Concept explainers
(a)
Interpretation:
The most important Lewis structure of
Concept Introduction:
Lewis structures represent covalent bonds and describe valence electrons configuration of atoms. The covalent bonds are depicted by lines, and unshared electron pairs by pairs of dots. The sequence to write Lewis structure of some molecule is given as follows:
- The central atom is identified and various other atoms are arranged around it. This central atom so chosen is often the least electronegative.
- Total valence electrons is estimated.
- single bond is first placed between each atom pair.
- The electrons left can be allocated as unshared electron pairs or as multiple bonds around the right
symbol of the element to satisfy the octet (or duplet) for each atom. - Add charge on the overall structure in case of polyatomic cation or anion.
The formal charge on each atom in the Lewis structure can be calculated from the equation written as follows:
Here,
(a)
Explanation of Solution
Lewis structure possible for

Since the double and triple bonds can conjugate therefore delocalization occurs that results in various equivalent resonance structures as indicated below:
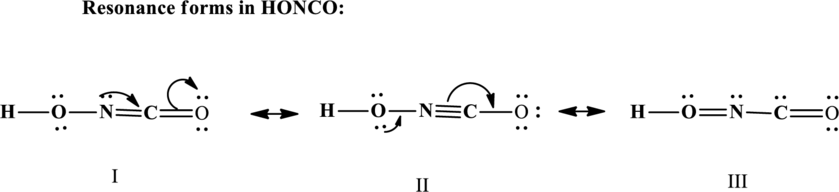
The formal charge on each atom in the Lewis structure is calculated from the equation as follows:
Substitute 5 for
Substitute 5 for
Substitute 4 for
Substitute 5 for
Substitute 4 for
Substitute 6 for
Substitute 6 for
Substitute 6 for
Substitute 1 for
Therefore the non-zero formal charges can be assigned as follows:
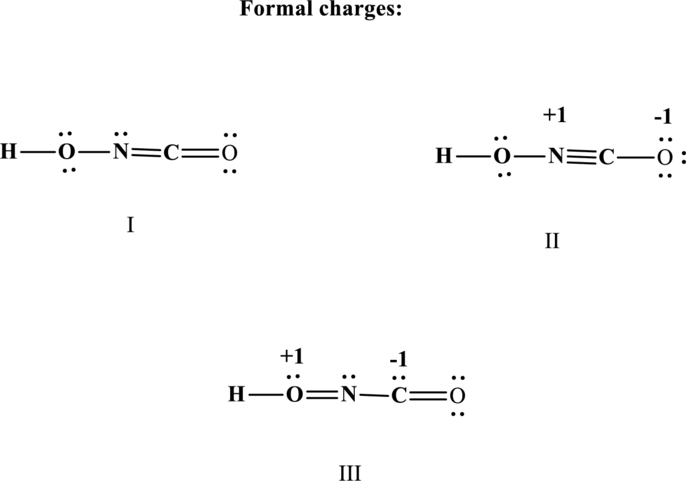
(b)
Interpretation:
The most important Lewis structure of
Concept Introduction:
Refer to part (a).
(b)
Explanation of Solution
Lewis structure possible for
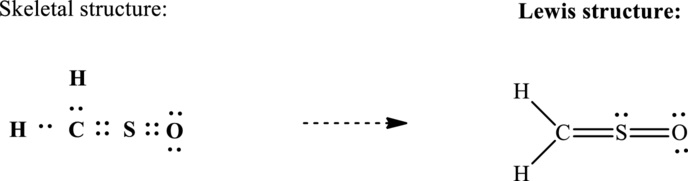
Since the double and triple bonds can conjugate therefore delocalization occurs that results in various equivalent resonance structures as indicated below:
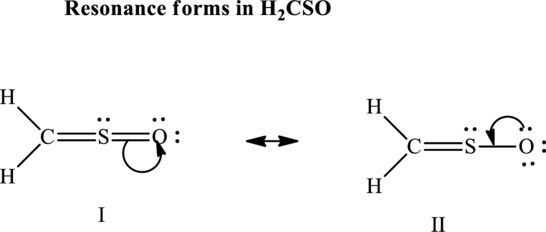
The formal charge on each atom in the Lewis structure is calculated from the equation as follows:
Substitute 6 for
Substitute 6 for
Substitute 6 for
Substitute 6 for
Substitute 1 for
Therefore the formal charges can be assigned as follows:
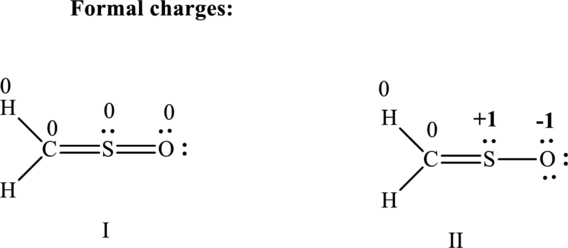
(c)
Interpretation:
The most important Lewis structure of
Concept Introduction:
Refer to part (a).
(c)
Explanation of Solution
Lewis structure for
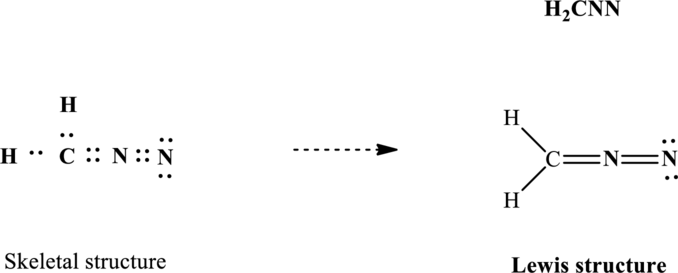
Since the double and triple bonds can conjugate therefore delocalization occurs that results in two equivalent resonance structures as indicated below:
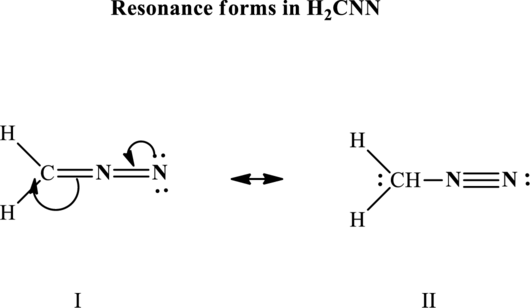
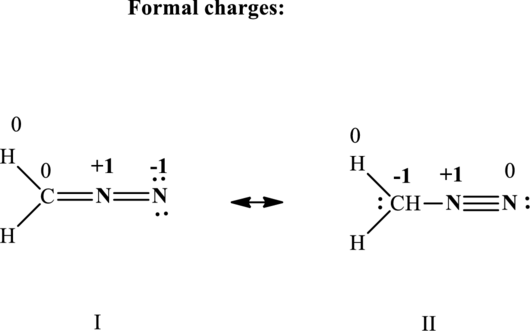
The formal charge on each atom in the Lewis structure is calculated from the equation as follows:
Substitute 5 for
Substitute 5 for
Substitute 5 for
Substitute 5 for
Substitute 4 for
Substitute 1 for
Therefore the formal charges in
(d)
Interpretation:
The most important Lewis structure of
Concept Introduction:
Refer to part (a).
(d)
Explanation of Solution
Lewis structure for
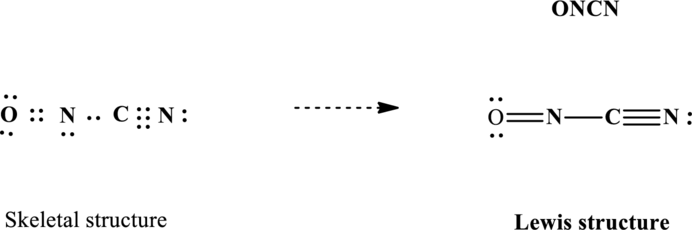
Since the double and triple bonds can conjugate therefore delocalization occurs that results in two equivalent resonance structures as indicated below:
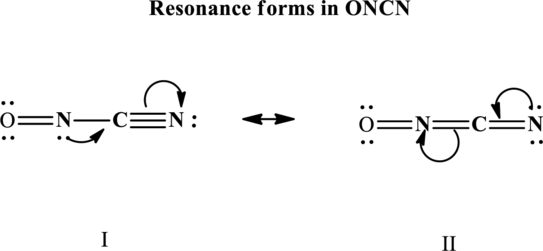
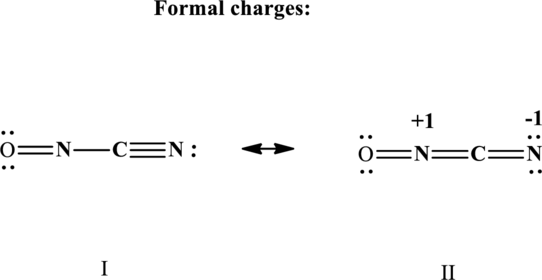
The formal charge on each atom in the Lewis structure is calculated from the equation as follows:
Substitute 5 for
Substitute 5 for
Therefore the non-zero formal charges in
Want to see more full solutions like this?
Chapter 2 Solutions
Chemical Principles: The Quest for Insight
- Consider the pyrosulfate ion, S2O72-. It has no sulfur–sulfur nor oxygen–oxygen bonds. (a) Write a Lewis structure for the pyrosulfate ion using only single bonds. (b) What is the formal charge on the sulfur atoms for the Lewis structure you drew in part (a)? (c) Write another Lewis structure using six bonds and two O—S bonds. (d) What is the formal charge on each atom for the structure you drew in part (c)?arrow_forwardFormamide, HC(O)NH2, is prepared at high pressures from carbon monoxide and ammonia, and serves as an industrial solvent (the parentheses around the O indicate that it is bonded only to the carbon atom and that the carbon atom is also bonded to the H and the N atoms). Two resonance forms (one with formal charges) can be written for formamide. Write both resonance structures, and predict the bond angles about the carbon and nitrogen atoms for each resonance form. Are they the same? Describe how the experimental determination of the HNH bond angle could be used to indicate which resonance form is more important.arrow_forwardWrite Lewis structures for these ions. Show all valence electrons and all formal charges. (Q) Acetate ion, CH3COO-arrow_forward
- 3.) Draw the two possible Lewis structures for acetamide, H₂CCONH2. Calculate the formal charge on each atom in each structure and use formal charge to indicate the more likely structure. Label all bonds as being polar or nonpolar.arrow_forwardBased on the atom connectivity shown bellow,evaluate the four resonance structure for the thiosulfate ion S2O3 ^2-. Use curved arrows to indicate how you get from one resonance structure to another. Assign formal changes to all atoms and determine which of these resonance structure is the most stable based on a formal charge analysis Explain your answer thoroughly. Look at the picture.arrow_forward1. Write the Lewis symbols for the following atoms:(a) Kr; (b) Ge; (c) N; (d) Ga; (e) Ace; (f) Rbarrow_forward
- Draw Lewis structures for each of the following ions. One atom in each ion has a formal charge that is not zero.Determine which atom it is, and what the formal charge is. (a) the C2H5 anion; (b) the CH3O cation; (c) the CH6N cation;(d) the CH5O cation; (e) the C3H3 anion (all three H atoms are on the same carbon)arrow_forward(a) Determine the formal charge of oxygen in the following structure. If the atom is formally neutral, indicate a charge of zero. (b) Draw an alternative Lewis (resonance) structure for the compound given in part (a). Show the unshared pairs and nonzero formal charges in your structure. Don't use radicals. Formal charge on O 0arrow_forwardDraw Lewis structures for each of the following molecules: (a) CH5N (contains a bond between C and N); (b) CH3NO2 (contains a bond between C and N but no bonds between C and O); (c) CH2O; (d) CH2Cl2; (e) BrCNarrow_forward
- A resonance hybrid is a structure that can be depicted by more than one valid Lewis structure. part1: Draw the major resonance form of fulminic acid, HCNO, with the atoms connected as indicated in the formula. Your structure should have nonzero formal charges minimized, and it should include all nonzero formal charges and all nonbonding electrons. part2: Draw the second most important resonance form of fulminic acid, HCNO, with the atoms connected as indicated in the formula. Your structure should have nonzero formal charges minimized, and it should include all nonzero formal charges and all nonbonding electrons. part3: Draw the least important resonance contributor for fulminic acid, HCNO, with the atoms connected as indicated in the formula. Your structure should have nonzero formal charges minimized and should include all nonzero formal charges and all nonbonding electrons.arrow_forwardWrite Lewis structures for the following: (c) C2F6 (contains a C¬C bond), (d) AsO3 3 -, (e) H2SO3 (H is bonded to O), (f) NH2Cl.. Arrange the bonds in each of the following sets in order of increasing polarity: (a) C¬F, O¬F, Be¬F; (b) O¬Cl, S¬Br, C¬P; (c) C¬S, B¬F, N¬O. What is the Lewis symbol for each of the following atoms or ions? (a) K, (b) As, (c) Sn2 + , (d) N3 Write electron configurations for the following ions and determine which have noble-gas configurations: (a) Cd2+, (b) P3-, (c) Zr4+arrow_forwardWrite Lewis structures for these ions. Show all valence electrons and all formal charges. (Q)Nitrate ion, NO3 -arrow_forward
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning


