
Concept explainers
(a)
Interpretation:
The class of given compound is to be identified.
Concept introduction:
The
Answer to Problem 43E
The class of compound
Explanation of Solution
The given organic compound is
Amines are the derivatives of ammonia. The formula of ammonia is
When the hydrogen of the ammonia is replaced by alkyl group amines is formed. The general formula of amines is shown below.
Therefore, the class of compound
The class of given has been rightfully identified.
(b)
Interpretation:
The class of given compound is to be identified.
Concept introduction:
The functional groups are atoms or the group of atoms that acts as substituents in the molecules and are responsible for their characteristic reactions. The functional group containing oxygen atoms are alcohol group, carboxylic acid group, aldehyde group, ketone group, phenols, ethers, and their derivatives.
Answer to Problem 43E
The class of compound
Explanation of Solution
The given organic compound is
Halogen is the elements that belong to the seventeenth group of the periodic table. When a halogen is combined with a hydrocarbon, then organic halides are formed. Fluorine is halogen.
Therefore, the class of compound
The class of given has been rightfully identified.
(c)
Interpretation:
The class of given compound is to be identified.
Concept introduction:
The functional groups are atoms or the group of atoms that acts as substituents in the molecules and are responsible for their characteristic reactions. The functional group containing oxygen atoms are alcohol group, carboxylic acid group, aldehyde group, ketone group, phenols, ethers, and their derivatives.
Answer to Problem 43E
The class of the given compound is ester.
Explanation of Solution
The structure of the given compounds is shown below.
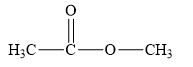
Figure 1
Esters are the derivatives of carboxylic acid. The general structure of carboxylic acid is shown below.
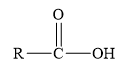
Figure 2
When the hydrogen of the hydroxyl group of carboxylic acid is replaced by another alkyl group, then ester is formed.
Therefore, the class of the given compound is ester.
The class of given has been rightfully identified.
(d)
Interpretation:
The class of given compound is to be identified.
Concept introduction:
The functional groups are atoms or the group of atoms that acts as substituents in the molecules and are responsible for their characteristic reactions. The functional group containing oxygen atoms are alcohol group, carboxylic acid group, aldehyde group, ketone group, phenols, ethers, and their derivatives.
Answer to Problem 43E
The class of the given compound is a carboxylic acid.
Explanation of Solution
The structure of the given organic compound is shown below.
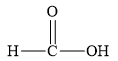
Figure 3
The given compound can release
The given compound is an organic acid.
Therefore, the class of the given compound is carboxylic acid.
The class of given has been rightfully identified.
(e)
Interpretation:
The class of given compound is to be identified.
Concept introduction:
The functional groups are atoms or the group of atoms that acts as substituents in the molecules and are responsible for their characteristic reactions. The functional group containing oxygen atoms are alcohol group, carboxylic acid group, aldehyde group, ketone group, phenols, ethers, and their derivatives.
Answer to Problem 43E
The class of the given compound is aldehyde.
Explanation of Solution
The structure of the given organic compound is shown below.
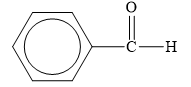
Figure 4
The carbonyl compounds are the compounds that contain
Therefore, the class of the given compound is aldehyde.
The class of given has been rightfully identified.
(f)
Interpretation:
The class of given compound is to be identified.
Concept introduction:
The functional groups are atoms or the group of atoms that acts as substituents in the molecules and are responsible for their characteristic reactions. The functional group containing oxygen atoms are alcohol group, carboxylic acid group, aldehyde group, ketone group, phenols, ethers, and their derivatives.
Answer to Problem 43E
The class of given compound is ether.
Explanation of Solution
The structure of the given organic compound is shown below.
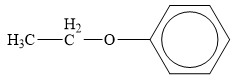
Figure 5
Ether is the derivatives of alcohol. The general formula of alcohols is shown below.
When the hydrogen of the alcohol is replaced by another alkyl group ether is formed.
Therefore, the class of given compound is ether.
The class of given has been rightfully identified.
(g)
Interpretation:
The class of given compound is to be identified.
Concept introduction:
The functional groups are atoms or the group of atoms that acts as substituents in the molecules and are responsible for their characteristic reactions. The functional group containing oxygen atoms are alcohol group, carboxylic acid group, aldehyde group, ketone group, phenols, ethers, and their derivatives.
Answer to Problem 43E
The class of given compound is ketone.
Explanation of Solution
The structure of the given organic compound is shown below.
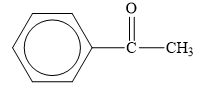
Figure 6
The carbonyl compounds are the compounds that contain
Therefore, the class of the given compound is ketone.
The class of given has been rightfully identified.
(h)
Interpretation:
The class of given compound is to be identified.
Concept introduction:
The functional groups are atoms or the group of atoms that acts as substituents in the molecules and are responsible for their characteristic reactions. The functional group containing oxygen atoms are alcohol group, carboxylic acid group, aldehyde group, ketone group, phenols, ethers, and their derivatives.
Answer to Problem 43E
The class of the given compound is amide.
Explanation of Solution
The structure of the given compounds is shown below.
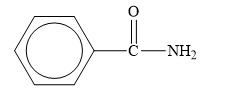
Figure 7
Amides are the derivatives of carboxylic acid. The general structure of carboxylic acid is shown below.
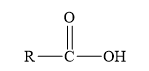
Figure 2
When the hydroxyl group of a carboxylic acid is replaced by amine group, then amide is formed.
Therefore, the class of the given compound is amide.
The class of given has been rightfully identified.
Want to see more full solutions like this?
Chapter 19 Solutions
Introductory Chemistry: Concepts and Critical Thinking (8th Edition)
- Following is the structural formula of acetylsalicylic acid, better known by its common name aspirin. (a) Name the two oxygen-containing functional groups in aspirin. (b) What is the molecular formula of aspirin?arrow_forward(a) The compound given below had the following IUPAC name and structural formula dibromocyclopentane C3H6CHBrCHBr (i) What type of isomerism is possible in the organic compound? (ii) Draw all the pairs of possible isomers and name them.arrow_forwardWrite two complete, balanced equations for each of the following reactions, one using condensed formulas and one using Lewis structures.(a) 2-butene reacts with chlorine.(b) benzene burns in air.arrow_forward
- Pentane and pentene. (a) Are isomers because they have the same molecular formula. (b) Are isomers because they have the same number of carbon atoms. (c) Are not isomers because they have different molecular formulas (d) Are not isomers because they have different namesarrow_forward(a) What is meant by the term isomer ? (b) Among the four alkanes, ethane, propane, butane, and pentane, which is capable of existing in isomeric forms?arrow_forwardDrawthe characteristic functional group of FOUR of the following six families of organic compounds: Alcohol, Amine, Aldehyde, Ketone, Carboxylic acid, or Ester.arrow_forward
- (a) Which of the following compounds, if any, is an ether? (b) Which compound, If any, is an alcohol? (c) Which com- pound, if any, would produce a basic solution if dissolved in water? (Assume solubility is not a problem). (d) Which compound, if any, is a ketone? (e) Which compound, if any, is an aldehyde? () Н,С—CH;—он Н (ii) H;C-Ñ-CH,CH=CH2 (ii) o (iv) (v) CH;CH,CH,CH2CHO (vi) CH3C=CCH,COOHarrow_forwardMany naturally occurring compounds contain more than one functional group. Identify the functional groups in the following compounds:(a) Penicillin G is a naturally occurring antibiotic.(b) Dopamine is the neurotransmitter that is deficient in Parkinson’s disease.(c) Capsaicin gives the fiery taste to chili peppers.(d) Thyroxine is the principal thyroid hormone.(e) Testosterone is a male sex hormone.arrow_forwardDraw astructural formula for each of the following compounds: (a)2-methylpropane (b)2,2-dimethylheptane c)2-methylpentanearrow_forward
- The following are incorrect names. Draw the compound and name it correct. (a) 1-chloro-2-bromopropane (b) 2,2-diethylbutanearrow_forwardWrite the chemical formula and Lewis structure of the following, each of which contains five carbon atoms:(a) an alkane(b) an alkene(c) an alkynearrow_forwardClassify each of he following hydrocarbons as alkanes, alkenes, or alkynes. (a) C6H14 (b) C3H4 (c) C9H18arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





