
Concept explainers
(a)
Interpretation:
The concept map is to be drawn and the volume of sulfur trioxide
Concept introduction:
A mole of a substance is defined as the same number of particles of the substance as present in
Answer to Problem 22E
The mole concept map is shown below.
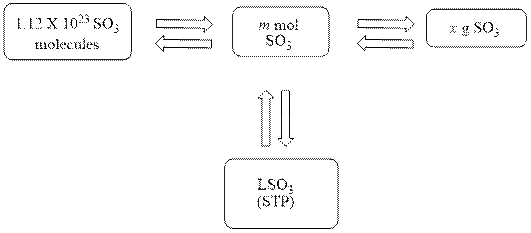
The liters of
Explanation of Solution
It is given that the number of molecules of
The mole concept map is shown below.

Figure 1
The Figure 1 shows that the number of moles should be calculated first, to calculate other parameters.
The number of moles is calculated from the relation shown below.
Therefore, the number of moles for
The volume of
The number of moles of
The volume for
The volume of
The mole concept map is shown Figure 1. The volume of
(b)
Interpretation:
The mole concept map is to be drawn and the mass of
Concept introduction:
A mole of a substance is defined as the same number of particles of the substance as present in
Answer to Problem 22E
The mole concept map is drawn below and the grams of
Explanation of Solution
It is given that the number of molecules of
The mole concept map is drawn below.

Figure 1
The above diagram shows that the number of moles should be calculated first, to calculate other parameters.
The number of moles is calculated from the relation shown below.
Therefore, the number of moles for
The molar mass of oxygen is
The molar mass of sulfur is
The mass of
The number of moles of
The mass for
Therefore, the mass of
The mass of
(c)
Interpretation:
The mole concept map is to be drawn and the molar concentration of
Concept introduction:
A mole of a substance is defined as the same number of particles of the substance as present in
Answer to Problem 22E
The mole concept map is drawn below and the molar concentration of
Explanation of Solution
It is given that the number of molecules of
The mole concept map is shown below.

Figure 1
The above diagram shows that the number of moles should be calculated first, to calculate other parameters.
The number of moles is calculated from the relation shown below.
Therefore, the number of moles for
The number of moles for of
The formula to determine molarity is shown below.
Where
•
•
•
Substitute the value of number of moles as
Therefore, the molar concentration of
The molar concentration of
Want to see more full solutions like this?
Chapter 15 Solutions
Introductory Chemistry: Concepts and Critical Thinking (8th Edition)
- (e) a pure substance QUESTION 6 Write chemical equations for each of the following chemical and physical processes: (a) Neutralization of an aqueous solution of barium hydroxide by the hydronium ion (b) Reaction of 1 mole of aluminum with I2(s) to form aluminum iodide (c) Conversion of 1 mole of O2(g) to O3(g) (d) Dissolving C12H22011(s) (sugar) in water (e) Combustion of CH3OH(t) (f) Thermal decomposition of 1 mole of solid sodium azide to produce solid sodium and nitrogen gas (g) Photodissociation of hydrogen gas (h) Fusion of gold QUESTION 7 Convert the units below Use dimensional analysis where appropriatearrow_forward1. How would each of the following procedural errors affect the value obtained for the molar volume of hydrogen gas? Explain your reasoning. (a) Some bubbles of hydrogen gas remained clinging to the sides of the tube. (b) Some magnesium metal was left un-reacted at the end of the experiment. (c) 7 mL of the HCl were used instead of 5 mL.arrow_forwardQ2) Choose the correct answer for Five of the following sentences: - 1- What are the conditions for gas like Carbon monoxide to obey the ideal gas laws? a- low temperature and low pressure b- low temperature and high pressure c- high temperature and low pressure d- high temperature and high pressure 2- How many moles of HCl are there in 10 mL of a solution with a concentration of 0.5 mol L-1? b- 0.05 mol а- 5 mol C- 0.5 mol d-1 mol 3- A sample of oxygen occupies 47.2 liters under a pressure of 1240 torr at 250C. What volume would it occupy at 250C if the pressure were decreased to 730 torr? a- 27.8 L b- 32.3 L c- 47.8 L d- 80.2 L 4- What is the molecular weight of a pure gaseous compound having a density of 4.95 g/L at - 35 oC and 1020 torr? а- 24 b-11 b- 72 d-120 5- What is the Temperature of a 2 liter/mole ideal gas with pressure 10 atm? a-243.6 K c- 289.1 K b- 268.4 K d- 313.5 K 6- 88 grams of CO2 at 27°C is applying 5 atm pressure, what is the volume occupied by it? a- 5.6 liter c-…arrow_forward
- (a) A student has a 10.0g sample of CaBr2. Show the setup of the calculation to determine the number of moles of CaBr2 in the sample. Include units in the setup. (You do not need to do any calculations.)arrow_forwardThe mineral galena is composed of lead(II) sulfide and has an average density of7.46 g/cm3. (a) How many moles of lead(II) sulfide are in 8.26 ft3 of galena?_x10^_ moles. (Enter your answer in scientific notation.) (b) How many lead atoms are in 5.93 dm3 of galena? _x10_ atoms of Pb (Enter your answer in scientific notation.)arrow_forwardHow many milliliters of 0.372 M NH4Cl will contain the following? (answer in scientific notation) (a) 0.52 mol NH4Cl (b) 31.8 g NH4Cl (c) 1.05 x 1020 formula units of NH4Clarrow_forward
- Gallium chloride is formed by the reaction of 2.6 L of a 1.44 M solution of HCl according to the following equation: 2Ga + 6HCl ⟶ 2GaCl3 + 3H2 . (a) Outline the steps necessary to determine the number of moles and mass of gallium chloride.arrow_forwardGallium chloride is formed by the reaction of 2.6 L of a 1.44 M solution of HCl according to the following equation: 2Ga + 6HCl ⟶ 2GaCl3 + 3H2 . (a) Outline the steps necessary to determine the number of moles and mass of gallium chloride. (b) Perform the calculations outlined. I2 is produced by the reaction of 0.4235 mol of CuCl2 according to the following equation: 2CuCl2 + 4KI ⟶ 2CuI + 4KCl + I2 . (a) How many molecules of I2 are produced? (b) What mass of I2 is produced? A student isolated 25 g of a compound following a procedure that would theoretically yield 81 g. What was his percent yield? A sample of 0.53 g of carbon dioxide was obtained by heating 1.31 g of calcium carbonate. What is the percent yield for this reaction? CaCO3 (s) ⟶ CaO(s) + CO2 (s) The phosphorus pentoxide used to produce phosphoric acid for cola soft drinks is prepared by burning phosphorus in oxygen. (a) What is the limiting reactant when 0.200 mol of P4 and 0.200 mol of O2 react according to P4 + 5O2 ⟶ P4…arrow_forwardAnswer the following questions by using mole concept. (a) Calculate the molarity of a 125 g Na2SO4 in 560 ml solution. [Atomic mass: Na=23 g/mol, S= 32 g/mol, O=16 g/mol, C=12 g/mol, H= 1 g/mol] (a1)no of moles of Na2SO4 ? (a2) molarity of solution? (b) 30 g C6H12O6 is dissolved in 550 g of water. Find out the molality of the solution. (b1) no of moles of C6H12O6 ? (b2) molality of solution? (c) How much volume of 1.5 M NaOH solution can be made by diluting 60 ml of 4 M NaOH? (d) Aspirin (C9H8O4) is a medication used to reduce pain, fever, or inflammation. Find out the mass of 5x1023molecules of aspirin in gram. (d1) no of moles of Aspirin? (d2) mass of aspirin?arrow_forward
- A sample of concentrated nitric acid has a density of1.41 g/mL and contains 70.0% HNO₃by mass.(a) What mass of HNO₃ is present per liter of solution?(b) What is the molarity of the solution?arrow_forward10. An element has two isotopes of relative atomic masses 41.0 u and 44.0 u. The relative abundance of the isotopes are 90.0% and 10.0% respectively. The average atomic mass of the element is: (A) 41.0u (B) 41.3u (C) 42.5u (D) 43.7u 2 11. Which of the following phrases best describes the term solubility? (A) the ability of a solvent to dissolve in a solute (B) the ability of a solute to dissolve in a solvent (C) the maximum amount of solute that will dissolve in solvent at a given temperature (D) the maximum amount of solvent that will dissolve in a solute at a given temperature 4arrow_forward12. A 6.35 gram sample of a mixture containing sodium carbonate is found to react completely with 75.0 mL of 1.25 M hydrochloric acid. The other components of the mixture did not react. The products of the reaction between the sodium carbonate and hydrochloric acid were sodium chloride, carbon dioxide, and water. (a) What was the percent by mass of sodium carbonate in the original mixture? (b) How many liters of carbon dioxide would have been generated if it had been collected at STP?arrow_forward
 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
