
Concept explainers
(a)
Interpretation:
The mole concept map is to be drawn. The volume of hydrogen sulfide
Concept introduction:
A mole of a substance is defined as the same number of particles of the substance as present in
Answer to Problem 21E
The mole concept map is shown below.
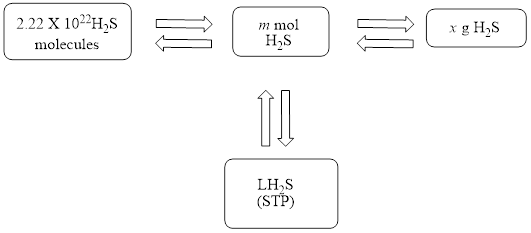
The volume of
Explanation of Solution
It is given that the number of molecules of
The mole concept map is shown below.

Figure 1
The Figure 1 shows that the number of moles should be calculated first, to calculate other parameters.
The number of moles is calculated from the relation shown below.
Therefore, the number of moles for
The volume of
The number of moles of
The volume for
Therefore, the volume of
The mole concept map is shown in Figure 1. The volume of
(b)
Interpretation:
The mole concept map is to be drawn. The mass of
Concept introduction:
A mole of a substance is defined as the same number of particles of the substance as present in
Answer to Problem 21E
The grams of
Explanation of Solution
It is given that the number of molecules of
The mole concept map is shown below.

Figure 1
The Figure 1 shows that the number of moles should be calculated first, to calculate other parameters.
The number of moles is calculated from the relation shown below.
Therefore, the number of moles for
The molar mass of hydrogen is
The molar mass of sulfur is
The mass of
The number of moles of
The mass for
Therefore, the mass of
The mass of
(c)
Interpretation:
The mole concept map is to be drawn and the molar concentration of
Concept introduction:
A mole of a substance is defined as the same number of particles of the substance as present in
Answer to Problem 21E
The molar concentration of
Explanation of Solution
It is given that the number of molecules of
The mole concept map is shown below.

Figure 1
The Figure 1 shows that the number of moles should be calculated first, to calculate other parameters.
The number of moles is calculated from the relation shown below.
Therefore, the number of moles for
The number of moles of
The formula to determine molarity is shown below.
Where
•
•
•
The relation between
The probable unit factors are given below.
The unit factor to determine
Therefore, the volume in
Substitute the value of number of moles as
Therefore, the molar concentration of
The molar concentration of
Want to see more full solutions like this?
Chapter 15 Solutions
Introductory Chemistry: Concepts and Critical Thinking (8th Edition)
- reaction (e) a pure substance QUESTION6 Write chemical equations for each of the following chemical and physical processes: (a) Neutralization of an aqueous solution of barium hydroxide by the hydronium ion (b) Reaction of 1 mole of aluminum with I2(s) to form aluminum iodide (c) Conversion of 1 mole of O2(g) to O3(g) (d) Dissolving C12H22011(s) (sugar) in water (e) Combustion of CH3OH(t) (f) Thermal decomposition of 1 mole of solid sodium azide to produce solid sodium and nitroge gas (g) Photodissociation of hydrogen gas (h) Fusion of goldarrow_forwardIf .30 mol of CuCO3 dissolved in 120 ml of water, what is the molarity of the solution?arrow_forwardDetermine the amount of sucrose in each solution.(a) 48 g of a solution containing 3.7% sucrose by mass(b) 103 mg of a solution containing 10.2% sucrose by mass(c) 3.2 kg of a solution containing 14.3% sucrose by massarrow_forward
- (e) a pure substance QUESTION 6 Write chemical equations for each of the following chemical and physical processes: (a) Neutralization of an aqueous solution of barium hydroxide by the hydronium ion (b) Reaction of 1 mole of aluminum with I2(s) to form aluminum iodide (c) Conversion of 1 mole of O2(g) to O3(g) (d) Dissolving C12H22011(s) (sugar) in water (e) Combustion of CH3OH(t) (f) Thermal decomposition of 1 mole of solid sodium azide to produce solid sodium and nitrogen gas (g) Photodissociation of hydrogen gas (h) Fusion of gold QUESTION 7 Convert the units below Use dimensional analysis where appropriatearrow_forwardWhat percent of water is in the compound Magnese (ii) chloride tetrahydrate?arrow_forwardWhat is the molar concentration of a lidocaine solution prepared by diluting 10. mL of a 2.0 M lidocaine stock solution to the mark in a 25 mL volumetric flask? Include units.arrow_forward
- 14) Which of the following terms best describes a carbonated beverage?(a) compound (b) heterogeneous mixture(c) homogeneous mixture (d) substance(e) none of the above show step solutions 15) Alum is used in styptic pencils to stop minor bleeding. If the formula is Al2(SO4)3 what is the total number of atoms in one formula unit of alum?(a) 10 (b) 12 (c) 14 (d) 17 (e) 2116) Using atomic notation, indicate the isotope having 25 p+, 30 n0,and 25 e-a. 2580Mn b. 2555Mn c. 5525Mn d. 2555Zn e. 3055Zn Show step by step solutions 17) Element X has two natural isotopes: X-6 (6.015 amu) and X-7 (7.016 amu).Calculate the atomic mass of element X given the abundance of X-7 is 92.5%.(a) 6.09 amu (b) 6.50 amu(c) 6.52 amu (d) 6.94 amu(e) 12.5 amu Show step by step solutions 18) Which of the following photons of visible light is most energetic?(a) blue (b) red(c) violet (d) yellow(e) all visible light photons have the same energy Show step by step solutions 19) Which element has the following…arrow_forwardTrue or false the following illustration correctly represents aqueous magnesium chloride.arrow_forwardSea water contains roughly 28.0 g of sodium chloride NaCl per liter. What is the molarity of sodium chloride in sea water?arrow_forward
- ) Solution Concentration and Solution Stoichiometry a) What is the molarity of a solution made by dissolving 6.08g of sodium acetate, CH;COONA, in water, and diluting it to a total volume of 0.750L? onal usearrow_forwardSECTION POSTLABORATORY ASSIGNMENT 1. Calculate the theoretical percentage of water for the following hydrates. (a) manganese(II) monohydrate, MNSO4 • H,O (b) manganese(II) tetrahydrate, MnSO4 • 4H2O 2. An unknown hydrate, AC XH2O, has a mass of 1.000 g before heating, and 0.738 g after heating. What is the experimental percentage of water in the hydrate? ofebyl lo slumol If the anhydrous compound (AC) has a molar mass of 101 g/mol, what is the water of crystallization (X) and the formula for the hydrate (AC• XH2O)?phut o (Ieuoiteo 2 molleoimd Formula of hydrate AC. H20 Water of crystallizationarrow_forwardConcentrated hydrochloric acid is made by pimping hydrogen chloride gas into distilled water, if concentrated HCI contains 439 g of HCI per liter, what is the molarity?arrow_forward
 World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning

