
Concept explainers
a)
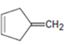
Interpretation:
Whether 4-methylenecyclopent-1-ene will have a π – π * UV adsorption in the 200 to 400nm range is to be stated.
Concept introduction:
Compounds having alternating double bonds (conjugated dienes) will have π – π *UV adsorption in the 200 to 400nm range.
To state:
Whether 4-methylenecyclopent-1-ene will have a π – π * UV adsorption in the 200 to 400nm range.
b)
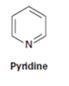
Interpretation:
Whether pyridine will have a π – π * UV adsorption in the 200 to 400nm range
Concept introduction:
Compounds having alternating double bonds (conjugated dienes) will have π – π * UV adsorption in the 200 to 400nm range.
To state:
Whether pyridine will have a π – π * UV adsorption in the 200 to 400nm range
c)
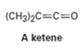
Interpretation:
Whether the ketene given will have a π – π * UV adsorption in the 200 to 400 nm range
Concept introduction:
Compounds having alternating double bonds (conjugated dienes) will have π – π * UV adsorption in the 200 to 400nm range.
To state:
Whether the ketene given will have a π – π * UV adsorption in the 200 to 400 nm range
Want to see the full answer?
Check out a sample textbook solution
Chapter 14 Solutions
Organic Chemistry
- For following substituted benzenes: [1] C6H5Br; [2] C6H5CN; [3] C6H5OCOCH3: Does the substituent activate or deactivate the benzene ring inelectrophilic aromatic substitution?arrow_forwardThe IR, and 1H NMR spectra of a compound with formula C5H6O2 are shown below. Determine the structure of the compound. You must fill all the parts to this question in the given spaces.arrow_forward1) A compound with molecular formula C49H96 could not be a ... 3)Compound Y has molecular formula C4H8O and its IR spectrum shows no absorption above 3000 cm-1 and no absorption between 1680 and 1840cm-1. Which of the following could be compound Y? 4) Which of the following will have its carbonyl absorption in its IR spectrum at the lowest wavelength? Please explain 1,3,4 thank youarrow_forward
- An unknown compound (whose molecular formula is C5H8O) shows IR absorption at 3600 and 3300 cm^-1. It’s ^1H-NMR spectrum contained singlets at delta 1.5, 2.2, and 2.9 (broad) ppm, in the ratio 6:1:1. Draw the structure for the unknown compound.arrow_forwardWe have no way of predicting whether the reagent shown will add to the Re or Si face of the aldehyde functional group. Which of the following is true about the reaction shown? CH, CH3 LIC CH OH CH The reaction can produce a mixture of R,R; S,S; R,S; and S,R isomers. The reaction may yield a mixture of R,S and R,R diastereomers. The reaction may create a mixture of S,R and S,S diastereomers. O The reaction will yield either R,R or S,S enantiomers.arrow_forwardDraw the bond-line (skeletal) structure of the compound with molecular formula C₂H₁3Br that gives the following alkene as the exclusive product of E2 elimination. Click and drag to start drawing a structure. C X C Ś c+arrow_forward
- A compound has the molecular formula C6H12O2. Its IR spectrum shows a strong absorption band near 1740 cm-1. Its 1H NMR spectrum consists of two singlets at δ 1.2 and δ 3.6. Which is the most likely structure of the compound?arrow_forward19. Specify whether you expect the benzene rings in the following compounds to be activated or deactivated. NH₂ NO₂ b) F NO₂ OH NO₂ NO₂arrow_forward4. Which of the following compounds will not show optical activity? a) CH2CICHBrCI b) CHзCH(ОH)СНз с) CH-CH-CHCICНЗ d) CH:(ОH)CH(NH-)COОHarrow_forward
- For the substituted cyclohexane compound shown, identify the atoms that are trans to the bromo substituent. I CH3 Br J HD HCI НА FF HB НОЕ HG CI Identify the atoms that are trans to the bromine. A В D E F H Karrow_forward1. At what position and on what ring would you expect the following substances to undergo electrophilic substitution? (b) CH3 Br lel CH3 2. Rank the compounds in each group according to their reactivity toward electrophilic substitution. (a) Chlorobenzene, o-dichlorobenzene, benzene (b) p-Bromonitrobenzene, nitrobenzene, phenol (c) Fluorobenzene, benzaldehyde, 0-xylene (d) Benzonitrile, p-methylbenzonitrile, p-methoxybenzonitrilearrow_forwardAn unknown compound has a molecular formula of C4H6O2. Its IR spectrum shows absorptions at 3095, 1762, 1254, and 1118 cm -1. It exhibits the following signals in its 1H NMR spectrum (ppm): 2.12 (singlet,3H), 4.55 (doublets of doublets, 1H), 4.85 (doublet of doublets, 1H), 7.25 (doublets of doublets, 1H); and the following signals in its 13C NMR spectrum (ppm): 20.8, 100.4, 141.2, 168.0. Draw the structure of the unknown compoundarrow_forward

 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning

