
Concept explainers
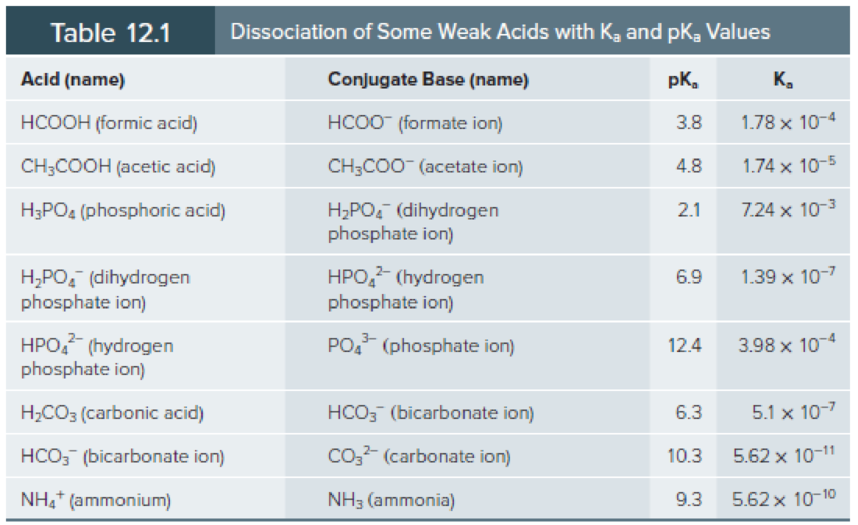
Use the Henderson-Hasselbalch equation and Table 12.1 to calculate the pH of the following solutions:
- a. 0.05 M formic acid and 0.1 M sodium formate.
- b. 0.2 M ammonium chloride and 0.1 M aqueous ammonia.
- c. 0.1 M acetic acid and 0.1 M sodium acetate.
(a)
Interpretation:
pH of the solution containing
Concept Introduction:
The
If the value of
Henderson-Hasselbalch equation:
Henderson-Hasselbalch equation explains the relationship between
Explanation of Solution
Given information are shown below,
pH of the solution can be determined using Henderson-Hasselbalch equation as given,
pH of the solution containing
(b)
Interpretation:
pH of the solution containing
Concept Introduction:
The
If the value of
Henderson-Hasselbalch equation:
Henderson-Hasselbalch equation explains the relationship between
Explanation of Solution
Given information are shown below,
pH of the solution can be determined using Henderson-Hasselbalch equation as given,
pH of the solution containing
(b)
Interpretation:
pH of the solution containing
Concept Introduction:
The
If the value of
Henderson-Hasselbalch equation:
Henderson-Hasselbalch equation explains the relationship between
Explanation of Solution
Given information are shown below,
pH of the solution can be determined using Henderson-Hasselbalch equation as given,
pH of the solution containing
Want to see more full solutions like this?
Chapter 12 Solutions
Chemistry In Context
- The acid dissociation constant of acetic acid in methanol is 3.02 × 10-¹0. Calculate the pH of the following solutions in acetic acid: 36. 0.035 M CH3COOH a. 3.10 b. 5.49 37. 0.035 M CH3COOH + 0.070 M CH3COONa a. 4.44 b. 5.05 38. 0.035 M CH3COONa a. 2.97 b. 4.32 c. 6.90 c. 9.22 c. 11.03 d. 8.51 d. 9.82 d. 12.38arrow_forwardConsider the generic dissociation equation... HA + H2O →H3O* + A¯ ..where HA is a weak acid Based on what you know about buffers, explain in detail how this reaction acts as an acid buffer to keep the pH of the solution constant even when a strong acid is added to the solution. Your answer should refer to the reaction above to discuss buffers, how they work, why strong acids/bases cannot be used as buffers, and how Le Chatelier's principle impacts why buffers work.arrow_forward6. At 25.0°C, 65.0 mL of 0.784 M ascorbic acid, HC6H7O6 was mixed with 45.0 mL of 0.371 M KOH. Ka = 8.1 x 10-5 a. Determine the moles of ascorbic acid b. Determine the moles of KOH C. Write out the equilibrium equation that forms and complete the ICE box. d. Determine the pH.arrow_forward
- Using the table of the weak base below, you have chosen Pyridine as your weak base in the buffer solution. You have already added enough of the conjugate acid salt to make the buffer solution concentration at 0.62 M in this salt. The desired pH of the buffer should be equal to 4.5. Values of K, for Some Common Weak Bases 車 Conjugate Acid Name Formula 1.8 x 10-5 4.38 x 10-4 5.6 x 10-4 3.8 x 10-10 Ammonia NH3 CH;NH2 CH§NH2 CH;NH2 C;H;N NH,+ Methylamine Ethylamine Aniline Pyridine CH;NH;* CH$NH;* CH;NH;* C;H;NH 1.7 x 10-9 1. Compute the pOH of the buffer solution. (Write your answer in 1 decimal place).arrow_forwardA formic acid buffer is prepared with 0.010 M each of formic acid (HCOOH) and Sodium formate (NaCOOH). The Ka for formic acid is 1.8 x 10-4. What is the pH of the solution? What is the pH if 0.0020 M of solid sodium hydroxide (NaOH) is added to a liter of buffer? What would be the pH of the sodium hydroxide solution without the buffer? What would the pH have been after adding sodium hydroxide if the buffer concentrations had been 0.10 M instead of 0.010 M?arrow_forwardA 1.00 liter solution contains 0.55 M nitrous acid and 0.41 M sodium nitrite. If 30 mL of water are added to this system, indicate whether the following statements are true or false. (Note that the volume MUST CHANGE upon the addition of water.) A. The concentration of HNO₂ will increase. B. The concentration of NO₂¯ will remain the same. ⒸC. The equilibrium concentration of H3O+ will increase. D. The pH will remain the same. [HNO₂] [NO₂-] True E. The ratio of will remain the same.arrow_forward
- Determine the concentration of H2SO4 when 10 mL of the surround water was collected near the C & H Sugar Company. Your sample was titrated with 5ml of 0.5 Molar NaOH solution to neutral the pH. Determine the concentration of H2SO4 in your water sample.arrow_forwardShown below is the pH curve for the titration of 0.30 M ethylamine (C2HSNH2(aq)) with 0.30 M HCI(aq). Determine the base ionization constant (KB) for ethylamine. A. 1.8 x 101" B. 6.2 x 102 C. 8.3 x 106 D. 5.6 x 104 E. 1.1 x 109 14 13 12 11 10 8. 1 10 20 30 40 50 60 volume of HCI added (mL) 9o7 6543 2 Hdarrow_forwardOnce upon a time, Tricia and her sister Jillian are doing titration to assess the molarity of the sample's total acid content. Using the pH measurements to report acidity, they were ordered to study a clear aqueous solution of an unknown monoprotic acid. They decided to use two methods of experiments to gather essential data for their Chemistry Class. The first method is through a pH strip. To estimate the pH of the sample, Tricia used a pH strip. Tricia collected 1 mL of the sample, which came out to have a pH of around 3.3. Furthermore, Tricia made a new setup in which 1 mL of the same sample was diluted with 9 mL of water, and the pH taken was now around 3.8. The second method is Titration, and Jillian used this method. Jillian prepared a 10 mL aliquot of the sample and diluted it with 25mL of distilled water. After this, Jillian added 2 drops of phenolphthalein, and it was titrated 3.54 mL of 0.048 mmol standardized NaOH to the endpoint. With this, answer the following questions.1.…arrow_forward
- Once upon a time, Tricia and her sister Jillian are doing titration to assess the molarity of the sample's total acid content. Using the pH measurements to report acidity, they were ordered to study a clear aqueous solution of an unknown monoprotic acid.They decided to use two methods of experiments to gather essential data for their Chemistry Class.The first method is through a pH strip. To estimate the pH of the sample, Tricia used a pH strip. Tricia collected 1 mL of the sample, which came out to have a pH of around 3.3. Furthermore, Tricia made a new setup in which 1 mL of the same sample was diluted with 9 mL of water, and the pH taken was now around 3.8.The second method is Titration, and Jillian used this method. Jillian prepared a 10 mL aliquot of the sample and diluted it with 25mL of distilled water. After this, Jillian added 2 drops of phenolphthalein, and it was titrated 3.54 mL of 0.048 mmol standardized NaOH to the endpoint.1. Using the data in the titration method,…arrow_forwardA 1.00 liter solution contains 0.28 M ammonia and 0.36 M ammonium chloride. If 0.180 moles of potassium hydroxide are added to this system, indicate whether the following statements are true or false. (Assume that the volume does not change upon the addition of potassium hydroxide.) A. The number of moles of NH3 will remain the same. B. The number of moles of NH4* will decrease. C. The equilibrium concentration of H₂O* will remain the same. D. The pH will increase. E. The ratio of (NH3)/ [NH4*] will increase. Submit Answer Retry Entire Group 5 more group attempts remainingarrow_forwardEqual quantities of 0.010M solutions of an acid HA and a base B are mixed. The pH of the resulting solution is 9.4 Part A:Write the equilibrium equation for the reaction between HA and B. Express your answer as a chemical equation. Identify all of the phases in your answer. Part B: Write equilibrium-constant expression for the reaction between HA and B. Part C: If Ka for HA is 8.0×10−5, what is the value of the equilibrium constant for the reaction between HA and B? Express your answer using one significant figure. Part D: What is the value of Kb for B? Express your answer using one significant figure.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





