
Concept explainers
(a)
Interpretation:
Structural formula of the ester formed by the reaction between acetic acid and n-propanol has to be drawn.
Concept introduction:
Ester: One
Ester formation: Esters are prepared by the reaction of a
Here, the
In chemistry, structure is the arrangement of
In structural formula,
- ✓ All of the atoms are shown at each end and intersection of bonds.
- ✓ H atoms are shown
(a)
Explanation of Solution
Esters are prepared by the reaction of a carboxylic acid and an alcohol molecule with the elimination of water molecule.
Here, the carboxylic acid is,
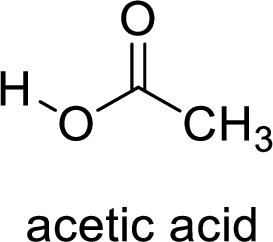
Alcohol is,
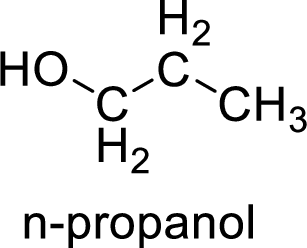
Here, the
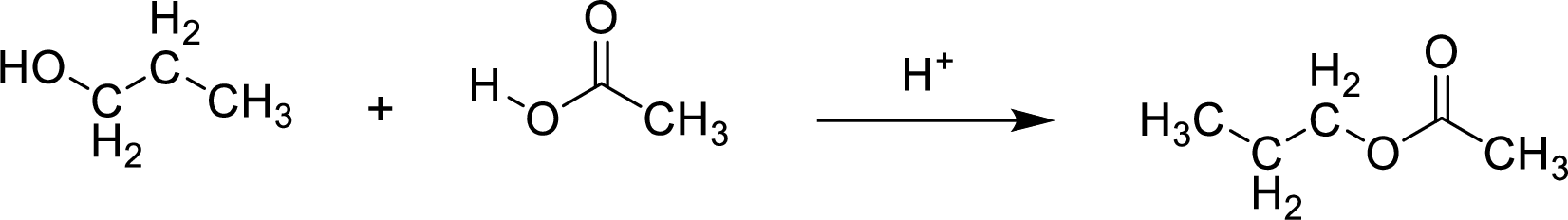
In structural formula,
- ✓ All of the atoms are shown at each end and intersection of bonds.
- ✓ H atoms are shown
Therefore,
The structural formulas for the ester formed is,
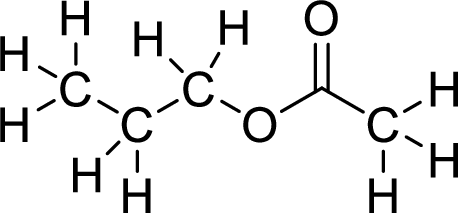
(b)
Interpretation:
Structural formula of the ester formed by the reaction between acetic acid and iso-propanol has to be drawn.
Concept introduction:
Ester: One
Ester formation: Esters are prepared by the reaction of a carboxylic acid and an alcohol molecule with the elimination of water molecule. The addition of a strong acid such as
Here, the
In chemistry, structure is the arrangement of chemical bonds between atoms in a molecule, specifically which atoms are chemically bonded to what other atoms with what kind of chemical bond.
In structural formula,
- ✓ All of the atoms are shown at each end and intersection of bonds.
- ✓ H atoms are shown
(b)
Explanation of Solution
Esters are prepared by the reaction of a carboxylic acid and an alcohol molecule with the elimination of water molecule.
Here, the carboxylic acid is,
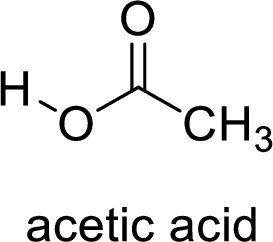
Alcohol is,
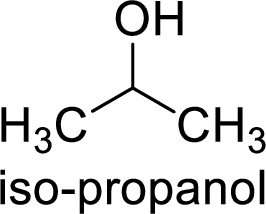
Here, the
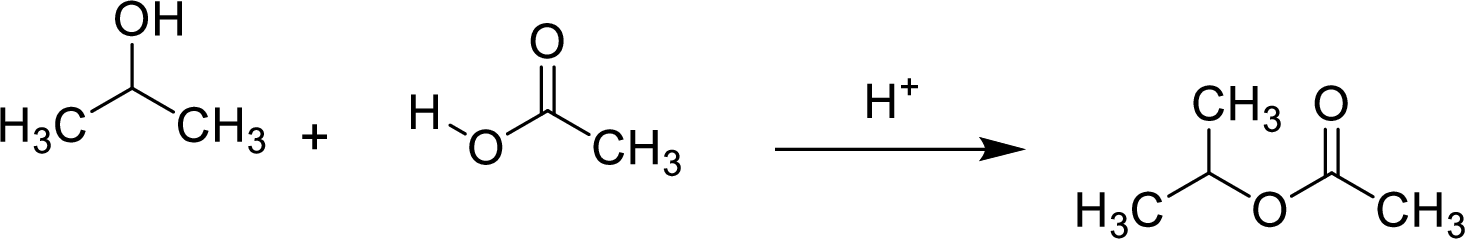
In structural formula,
- ✓ All of the atoms are shown at each end and intersection of bonds.
- ✓ H atoms are shown
Therefore,
The structural formulas for the ester formed is,
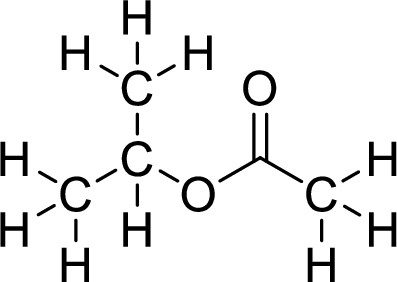
(c)
Interpretation:
Structural formula of the ester formed by the reaction between acetic acid and iso-propanol has to be drawn.
Concept introduction:
Ester: One
Ester formation: Esters are prepared by the reaction of a carboxylic acid and an alcohol molecule with the elimination of water molecule. The addition of a strong acid such as
Here, the
In chemistry, structure is the arrangement of chemical bonds between atoms in a molecule, specifically which atoms are chemically bonded to what other atoms with what kind of chemical bond.
In structural formula,
- ✓ All of the atoms are shown at each end and intersection of bonds
- ✓ H atoms are shown
(c)
Explanation of Solution
Esters are prepared by the reaction of a carboxylic acid and an alcohol molecule with the elimination of water molecule.
Here, the carboxylic acid is,
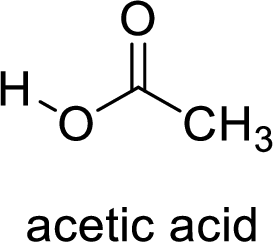
Alcohol is,
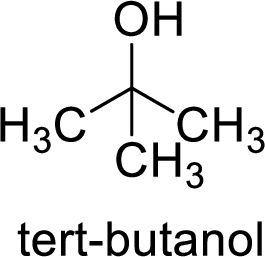
Here, the
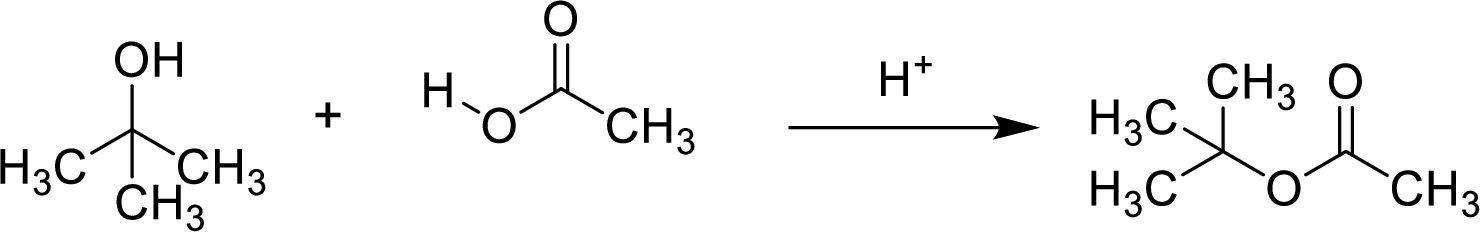
In structural formula,
- ✓ All of the atoms are shown at each end and intersection of bonds.
- ✓ H atoms are shown
Therefore,
The structural formulas for the ester formed is,
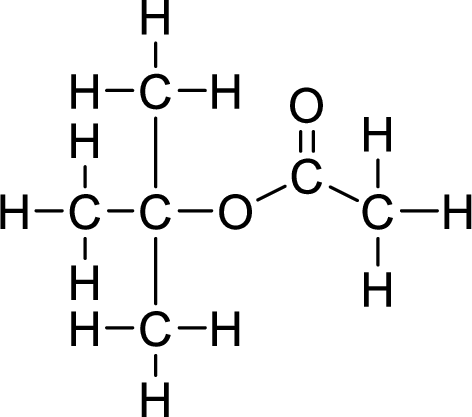
Want to see more full solutions like this?
Chapter 12 Solutions
Chemistry In Context
- Alcohols are very useful starting materials for the production of many different compounds. The following conversions, starting with 1-butanol, can be carried out in two or more steps. Show the steps (reactants/catalysts) you would follow to carry out the conversions, drawing the formula for the organic product in each step. For each step, a major product must be produced. (See Exercise 62.) (Hint: In the presence of H+, an alcohol is converted into an alkene and water. This is the exact reverse of the reaction of adding water to an alkene to form an alcohol.) a. 1-butanol butane b. 1-butanol 2-butanonearrow_forwardketone a. carbonyl group carboxylic acid b. hydroxyl group 3. alkane c. general formula R3N 4. alkyne d. triple bond 5. ester e. smell of fruits 6. alcohol f. fuels amine g. vinegar 8. hydrocarbon h. carbon and hydrogenarrow_forwardA. Identify the name of the functional group where the given organic compounds belong (alcohol, ether, aldehyde, ketone, carboxylic acid, ester, amine) 1. H H Br H H -C C-H H H H 2. H H Н—с H -5- H H N- H- 3. H H -нн I-arrow_forward
- Identify the organic functional groups and reaction type for the following reaction.The reactant is a(n)a. secondary amideb. carboxylic acidc. tertiary amided. aromatice. ketonef. primary amideg. aldehydeh. amineThe products are a(n)a. carboxylate ion and amineb. ketone and aminec. carboxylic acid and amided. carboxylic acid and alcohole. ester and aminef. aldehyde and amineg. carboxylic acid and ammonium ionThe reaction type isa. hydrolysis (in acid)b. amide synthesisc. hydrationd. esterificatione. dehydrationf. hydrolysis (in base)arrow_forward2. Draw the mechanism of the bromination of acetylene: 3. Draw the structures of the following: a. 3-bromo-3,4-dimethyl-1-hepten-5-yne b. Acetylene c. 1-ethynyl-2-methylcyclohexanearrow_forwardIdentify the organic functional group of the reactant and the reaction type then predict the functional group(s) of the product(s).The reactant is a(n)a. ketoneb. esterc. amided. alcohole. carboxylic acidf. amineg. aldehydeThe reaction type isa. hydrationb. hydrolysis (in base)c. esterificationd. dehydratione. hydrolysis (in acid)f. amide synthesisThe product should be a(n)a. ketoneb. aldehydec. alcohol onlyd. estere. carboxylic acid and an alcoholf. carboxylic acid onlyg. amidearrow_forward
- Predict the functional group of the product for the following reaction. a. ester b. carboxylic acid c. hemiacetal d. acetal e. amidearrow_forwardHO H₂C. CH O CH, H CH₂ ... Choose a match Alcohol Aldehyde Ketone Carboxylic acid Etherarrow_forwardAldehydes and ketones may be reduced to alkanes. O alcohols. esters. acids. ethers.arrow_forward
- 5. Draw a structural example of a primary, secondary and tertiary alcohol. 6. Classify the following reaction as oxidation, dehydration or hydration. a. CH.CH(OH)CH, CH,COCH, 7. Identify each compound as an alcohol or an ether. Classify any alcohols as primary, secondary or tertiary. он b. он HO d. 8. Write the product(s) for the following reactions. a. H,O b. H,Oarrow_forwardWhat is the name of this alcohol?a. 3-Ethylbutan-2-olb. 2-Ethylbutan-3-olc. 3-Methylpentan-2-old. 3-Methylpropan-4-olarrow_forward1. What functional group is produced when an aldehyde reacts with H2/Pt? A.secondary alcohol B. carboxylic acid C.hemiacetal D. primary alcohol E.alkane F.tertiary alcohol G. alkene 2. What reaction occurs when an aldehyde reacts with H2/Pt to form a primary alcohol? A. Hydration B. Hydration C. Dehydration D. Oxidation E. Reduction( hydrogentation) 3. What reaction occurs when an Ester react with H+/H2O to from a carboxylic acid and alcohol? A. Dehydration B. Reduction ( Hydrogenation) C.Hydrolysis D. Hydration E.oxidationarrow_forward
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning





