
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN: 9780618974122
Author: Andrei Straumanis
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 1, Problem 15CTQ
Interpretation Introduction
Interpretation:
The reason for not having an official shape as defined in Model 4 for molecule H-F should be stated.
Concept Introduction:
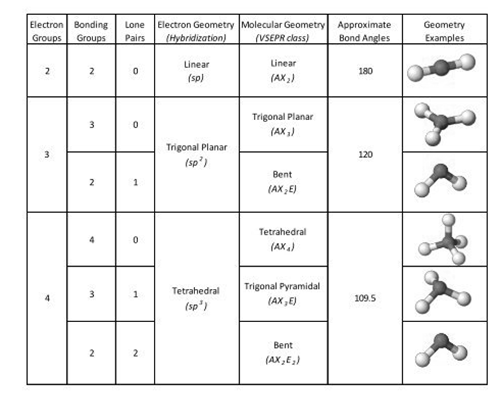
The angle which is described by the lines that joined the centers of two atoms to a third atom to which all atoms are covalently bonded is known as a bond angle.
In simple language, an angle that is defined by any three atoms in a molecule is known as a bond angle.
Valence shell electron pair repulsion theory is used to determine the geometry of different molecules from the electron pairs present around their central atoms.
The chart of VSEPR theory is shown below:
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Draw a Lewis structure for the molecule below, showing all lone pairs. You may abbreviate any methyl groups as CH,.
HOCH,CH,OCH;
Click and drag to start
drawing a structure.
answer 3 and 5. PLS MAKE IT LIKE THIS WAY SO THAT I UNDERSTAND IT
IONIC YES OR NO
POLAR YES OR NO
NON POLAR YES OR NO
IMF EXIST IS.....
NO EXPLANATION NEEDED.
Read This!
The attractive and repulsive forces in an atom are rather complex. An electron is attracted to the protons
in the nucleus, but it is also repelled by the other electrons in the atom. It is important to note however
that the attractive force of the nucleus is NOT divided up among the electrons in the atom. Each electron
gets approximately the full attractive force of the nucleus (minus the repulsive effects of other electrons).
Compare the diagram below to set D in Model 3. Notice the similarity in attractive force.
0.10 nm
0.10 nm
approx. 4.60 x 10-8
(on each electron)
Model 4 – Period 3 Elements
Aluminum
Chlorine
Sodium
What does it MEAN?
What do you WONDER?
What do you SEE?
Chapter 1 Solutions
Organic Chemistry: A Guided Inquiry
Ch. 1 - (E) What does the number (+Z) at the center of...Ch. 1 - Prob. 2CTQCh. 1 - Prob. 3CTQCh. 1 - Prob. 4CTQCh. 1 - Prob. 5CTQCh. 1 - Prob. 6CTQCh. 1 - Prob. 7CTQCh. 1 - You hear a student from a nearby group say that...Ch. 1 - Use VSEPR to explain why the HBH bond angle of BH3...Ch. 1 - Both the HCH and HCO bond angles of H2CO...
Ch. 1 - Prob. 11CTQCh. 1 - Consider the following flat drawing of methane...Ch. 1 - Use VSEPR to assign a value of (close to) 109.5,...Ch. 1 - A student draws the picture of ammonia (NH3) in...Ch. 1 - Prob. 15CTQCh. 1 - How many central atoms does the molecule H2NCH3...Ch. 1 - Indicate the bond angle and shape about each...Ch. 1 - Explain how there can be two kinds of bent:...Ch. 1 - A student makes the following statement: “The...Ch. 1 - A student who missed this class needs to know how...Ch. 1 - Prob. 1ECh. 1 - Prob. 2ECh. 1 - Consider the incomplete valence shell...Ch. 1 - How many valence electrons does a neutral a. K...Ch. 1 - Consider the molecules AlCl3 (aluminum chloride)...Ch. 1 - Draw an example of a bent molecule with a bond...Ch. 1 - Label each atom marked with an arrow with the...Ch. 1 - a model of each of the following molecules: a....
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- first. C3H + O,arrow_forwardCheck the box next to each molecule on the right that has the shape of the model molecule on the left: molecules model (check all that apply) O COCI, | CH;0 You can c CH4 O CH,Cl, O None of the above Note for advanced students: the length of bonds and size of atoms in the model is not necessarily realistic. Th geometry and 3D shape of the molecule.arrow_forwardPlease construct a I -C-E table.arrow_forward
- What is the working and solution to find the Carbon (marked with *) bond angle?arrow_forwardIn the boxes below. Draw 3 resonance structures for the molecule attached in the image. Then circle the best one out of all 3.arrow_forwardThe second picture is eq 5 which is needed to solve. Not another question just in case it gets flaggedarrow_forward
- For 1 and 2, use curved arrows to illustrate the potential overall electron movements or bond changes, and identify the type of reaction by examining the overall chemical transformation. Show A-H bonds as needed.arrow_forward(do last four asap )arrow_forward1.) Circle and label all of the non-alkane functional groups in the molecule shown below. Be as specific as possible (use 1o, 2o and 3o to describe amines and amides). 2.) Draw the line angle structure for the molecule in the box.arrow_forward
- Please make sure to answer all the below. Need the correct asnwer with attention to detail.arrow_forwardHelp, what is the name molecule?arrow_forwardDraw a complete structure for hydroxide ion, OH. Explicitly draw all H atoms. • Include all valence lone pairs in your answer. • In cases where there is more than one answer, just draw one. • Do not use the square brackets tool in your answer.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning