
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN: 9780618974122
Author: Andrei Straumanis
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 1, Problem 14CTQ
A student draws the picture of ammonia
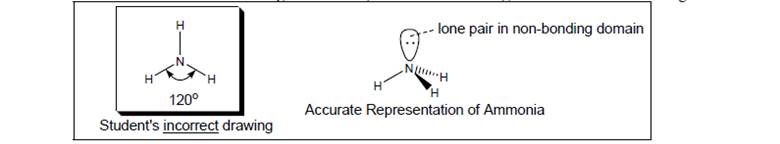
a. What did the student omit from his drawing?
b. What is the actual
c. Explain why water, ammonia, and methane (shown below) all have about the same bondangles (close to 109.5°) even though they have different numbers of bonds.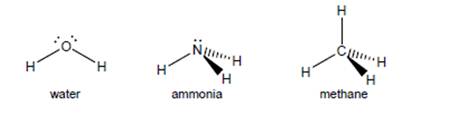
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Fill in the table. Central atom is listed first. A. Write the number of valence electrons below the formulaB. Draw the Lewis structureC & D. Write the Electron Group Geometry and Molecular Shape NamesE. Write the bond angleF. Write the molecular polarity. "P" for polar and "NP" for nonpolar.
SpeciesValenceElectrons(1 pt.)
LewisStructure(2 pt.)
Electron PairGeometryName(1 pt.)
Molecular ShapeName (1 pts.)
BondAngle (1 pt.)
Molecular Polarity(1 pt.)
PO43-
NOBr
Upload
For the formula CH2CF2, "draw the Lewis structure in your work (..
PAfter drawing the complete Lewis structure, type in the shape, bond angle, etc. for each
blank.
What is the molecular shape or geometry for the carbon on the left (the C in CH2)?
trigonal planer
What is the molecular shape or geometry for the carbon on the right (the C in CF2)?
trigonal planer
What is the bond angle for the carbon on the left (the C in CH2)? Type in a plain number, but
include a "<" sign in front of the number if applicable.
What is the bond angle for the carbon on the right (the C in CF2)? Type in a plain number, but
include a "<" sign in front of the number if applicable.
What is the hybridization for the carbon on the left (the C in CH2)? Type this in without any
superscript, for instance sp3 would be typed as "sp3".
What is the hybridization for the carbon on the right (the C in CF2)? Type this in without any
superscript, for instance sp would be typed as "sp3".
Is the molecule overall polar or…
Consider the molecules
— BrF5.
A. Draw the best Lewis structure for this molecule. Label any atoms with nonzero formal charge.
B. Label each bond angle. As part of your answer be sure to include if it is more or less than the ideal bond angle.
C. What is the electron geometry around the bromine atom?
D. Are the bonds in the molecule polar?
E. Is the overall molecule polar?
— CH2 F2 .
A. Draw the best Lewis structure for this molecule.
B. Label each bond angle. Answers for A-D here:
C. Redraw the shape of the molecule. Draw all dipoles.
D. Is the overall molecule polar?
— Consider the molecule CH2 CF2 .
A. Draw the best Lewis structure for this molecule.
B. Label each bond angle.
C. Redraw the shape of the molecule (according to the exacting specifications of your instructor). Draw all dipoles.
D. Is the overall molecule polar?
Chapter 1 Solutions
Organic Chemistry: A Guided Inquiry
Ch. 1 - (E) What does the number (+Z) at the center of...Ch. 1 - Prob. 2CTQCh. 1 - Prob. 3CTQCh. 1 - Prob. 4CTQCh. 1 - Prob. 5CTQCh. 1 - Prob. 6CTQCh. 1 - Prob. 7CTQCh. 1 - You hear a student from a nearby group say that...Ch. 1 - Use VSEPR to explain why the HBH bond angle of BH3...Ch. 1 - Both the HCH and HCO bond angles of H2CO...
Ch. 1 - Prob. 11CTQCh. 1 - Consider the following flat drawing of methane...Ch. 1 - Use VSEPR to assign a value of (close to) 109.5,...Ch. 1 - A student draws the picture of ammonia (NH3) in...Ch. 1 - Prob. 15CTQCh. 1 - How many central atoms does the molecule H2NCH3...Ch. 1 - Indicate the bond angle and shape about each...Ch. 1 - Explain how there can be two kinds of bent:...Ch. 1 - A student makes the following statement: “The...Ch. 1 - A student who missed this class needs to know how...Ch. 1 - Prob. 1ECh. 1 - Prob. 2ECh. 1 - Consider the incomplete valence shell...Ch. 1 - How many valence electrons does a neutral a. K...Ch. 1 - Consider the molecules AlCl3 (aluminum chloride)...Ch. 1 - Draw an example of a bent molecule with a bond...Ch. 1 - Label each atom marked with an arrow with the...Ch. 1 - a model of each of the following molecules: a....
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- On the left side of Figure 3.6, label the areas shown with a dotted line where... one bond can form. one bond can form.arrow_forwardHelp me pleasearrow_forwardA model for SiH4 is shown in the chem3D window. SiH4 has tetrahedral geometry. ball & stick |+ labels Rotate the molecule until you have a feeling for its three-dimensional shape. How many atoms are bonded to the central atom? If you take any three of the outer atoms, what shape do they define? | Consider the bond angles at the central atom. Do they all have the approximately the same numerical value? What is the approximate numerical value of this angle? | degrees. Are all four positions about the central atom equivalent, or is one of them different from the other three. For practice, type in the name of the geometry of the molecule: Previous Nextarrow_forward
- 3a.Draw the best Lewis structure for the HClO3 acid molecule. (Hint: the acidic H is bonded to O in an oxy-acid molecule).Evaluate each atom using formal charge to prove you have the best structure. Show your math work as well as your formal charge answers. 3b. Draw all the resonance structures for the ClO3– polyatomic anion. (Hint: make sure you have the best initial structure first!)arrow_forwardDraw the Lewis structure for PF5 in the window below and then answer the questions that follow. Is PF5 polar or nonpolar? C Sn [F ? ChemDoodlearrow_forward4. Write the Lewis dot (electron dot) symbol for each covalent molecule. Remember the number of unpaired electrons in the Lewis dot symbol of the atom determines the number of bonds each atom makes. If your molecule has unpaired electrons you are not done. If your molecule has the wrong number of bonds for some atoms it is wrong. Try a different arrangement of atoms. Working left to right will help you. Show loan pairs. a. HCN b. CH3COOH c. H₂CCONHCH3 d. HCCH e. C6H12arrow_forward
- What's More Activity 2: The Name Is Bond... Chemical Bond Directions: Fill out the table below with correct answers. The first one is done for you. Compound NaCl CH4 HCI N₂ 0₂ H₂O KBr MgClz PC|3 CO CaF₂ Type of Bond ionic Good conductor of heat or electricity? Yes High Boiling Point? Yes High Melting Point? Yesarrow_forwardFor the formula SO2, "draw the Lewis structure in your work" (wh it). After drawing the complete Lewis structure, type in the shape, bond angle, etc. for each blank. What is the molecular shape or geometry? What is the bond angle? Type in a plain number, but include a "<" sign in front of the number if applicable. What is the hybridization? Type this in without any superscript, for instance sp would be typed as "sp3". Is the molecule overall polar or nonpolar? Simply type "polar" or "nonpolar".arrow_forwardWhat is the nature of the bonding in C3H₂F2, Is it polar? A. Submit your drawing with dipole moments B. Identify the molecules polarity C. Identify the molecules geometriesarrow_forward
- 11 Name: Maddie Klink Instructor: Lab Day: Models of Organic Molecules Please bring your molecular model kits to lab for the completion of these exercises. Molecular Modeling: This lab is designed to help you become comfortable using model kits to visualize molecular structures in three dimensions. Part I: Answer these questions before doing any work with your model kit 1. How many bonds must each of the following atoms have in order to have no formal charge? 4 valence 1450na a. Carbon b. Hydrogen bond c. Oxygen d. Chlorine 7 valence e. Nitrogen 5 valence Octet H- H Complete octet = 8-4 H 8-S = 3 3bonds 2. Using the information from question #1, determine whether each of the following structures is possible or impossible? (Note that if no formal charges are shown, this implies that all formal charges are zero.) 6 valence Octet = 8.6 H H с-н H 01C C -K = 2 bonas 8-1-1 bond H H H-C Lab Time: H -C-H H H H-C-CI- C-H ++ H H -H HIC C-H H VEUR 1arrow_forwardDraw the Lewis structure for SbF3 in the window below and then answer the questions that follow. 0 . Do not include overall ion charges or formal charges in your drawing. + O 40 000 [F Submit Answer What is the electron-pair geometry for Sb in SbF3? What is the the shape (molecular geometry) of SbF3? ChemDoodle An error has been detected in your answer. Check for typos, miscalculations etc. before submitting your answer. Retry Entire Group 9 more group attempts remainingarrow_forwardHow many σ-bonds are in the molecule? σ-bonds How many π-bonds are in the molecule? π-bonds For bond angles, respond with 60o, 90o, 109o, 120o, or 180o (do not include the degree sign in your response). What is the approximate H-C-C bond angle in the molecule? o What is the approximate C-O-H bond angle in the molecule? o What is the approximate C-N-H bond angle in the molecule? o What is the approximate O=C-O bond angle in the molecule? oarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning
Stoichiometry - Chemistry for Massive Creatures: Crash Course Chemistry #6; Author: Crash Course;https://www.youtube.com/watch?v=UL1jmJaUkaQ;License: Standard YouTube License, CC-BY
Bonding (Ionic, Covalent & Metallic) - GCSE Chemistry; Author: Science Shorts;https://www.youtube.com/watch?v=p9MA6Od-zBA;License: Standard YouTube License, CC-BY
General Chemistry 1A. Lecture 12. Two Theories of Bonding.; Author: UCI Open;https://www.youtube.com/watch?v=dLTlL9Z1bh0;License: CC-BY