
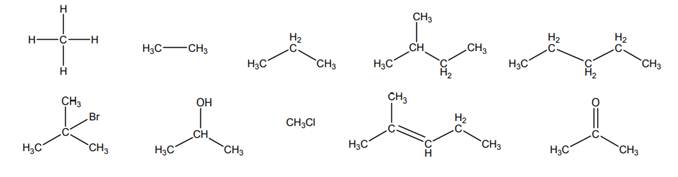
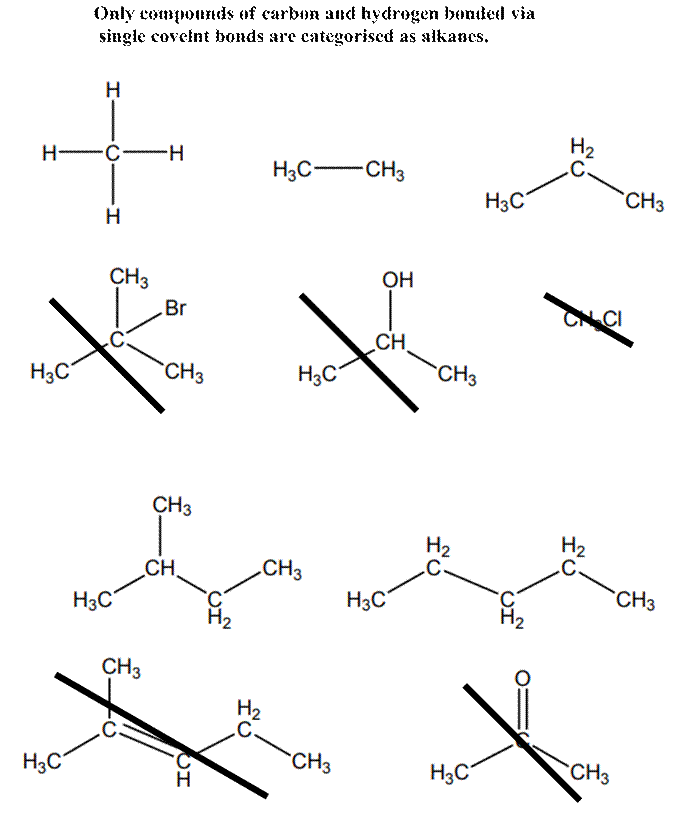
Interpretation: Molecules given below that does not represent an alkane should be crossed out.
Concept introduction:
Explanation of Solution
Alkanes are essentially pure hydrocarbons with no additional
Want to see more full solutions like this?
Chapter NW1 Solutions
Organic Chemistry: A Guided Inquiry
- Identify whether the compound is an alkane, alkane, alcohol, or ether. Note: I need answers immediately. I will send a good rating right away as well.arrow_forward...me the family to which each organic compound belongs. The first answer has been filled in for you. compound O || CH3- C- CH₂ - CH CH₂ || CH3 I CH₂ - CH CH₂ || OH I C || O O=CIC - H I CH3 CH₂=CH-OH family aldehydearrow_forward15. For the molecules:2,2-dimethylhexane, and octane. Which of the 2,3,4-trimethylpentane following structures will have the highest bp, lowest mp, and the least soluble in water?arrow_forward
- Identify whether the following is an alkane, alkyne, alcohol or ether. Note: I need answers immediately. Will send a good rate right away as well.arrow_forwardFor each of these cycloalkanes, which are partly skeletal (line) drawings, and also have two or three methyl groups attached to them, add the hydrogens which are not shown (the hydrogens on the methyl C's are already indicated as you see). Determine the molecular formula (C_H for each. H3C CH3 H;C. CH3 H3C- CH3 CH3 C 7 H C_ H C Harrow_forwardRewrite each of these structures as a condensed structure. H F\H H H-C-Ċ-Ċ –Ċ-H H FHH condensed structure: CH3CF2CH2CH3 H H нНН H. H-С—С—С—Н H O H. 1 H. condensed structure: CH-3-CH-CH3arrow_forward
- What is the name of the IUPAC compound shown here? (see attatchement) First time you guys answered it was wrong, trying again. **** The answer 3-methylbut-1-ene you gave yesterday was WRONG . Please help with correct answer. This is what the question has as well: Alkanes are called saturated hydrocarbons because each carbon atom has the maximum number of hydrogen atoms. In contrast, alkenes and alkynes are unsaturated because they contain double or triple bonds that reduce the number of hydrogen atoms in the compound. When naming unsaturated hydrocarbons, a suffix reflects the type of multiple bond in the compound: ene is used for alkenes (double bond), and yne is used for alkynes (triple bond). For cyclic alkenes and alkynes, the ring is numbered such that the double or triple bond is between the first two carbon atoms. For straight-chain molecules, the numbering starts at one end of the chain, so the location of the double or triple bond must be specified in the name.arrow_forwardA model of an alkane appears in the window below. ball & stick + labels Which of the following represent structural isomers of the molecule shown in the model? Choose all that apply. CHз CH3-CH-CH3 сH-CHa-CH2-CН2-CH3 .CH2 CH2 сCнa CH2 CH2 CHз CH3 -CHз CH3 сHз CH3-CH-CH2-CH3arrow_forwardA model of an alkane appears in the window below. ball & stick -+ labels Which of the following represent structural isomers of the molecule shown in the model? Choose all that apply. CH3 CH-CH-CH2-CHз ҫHз снз CH3-сH—ҫ-сн2-CHз CHз CH3-CH2-CH2-CH2-CH-CH3 ҫHз сна CH3-C-CH2-CH-CH3 CHз сHз снз CH3-C- CH-CH2-CH3 CHзarrow_forward
- A model of a cycloalkane appears in the window below. ball & stick + labels Which of the following represent structural isomers of the molecule shown in the model? Choose all that apply. n CH3-CHz-CH2-CH2-CH3 CH2. CCH-CH2-CH3 CH CH2. ссH—сHз CH CHз CH2 CH-CH3 сн CH2 CHз Cнз CH- CH2arrow_forward3. Name all the functional group/s in the following organic compound. This is the chemical compound 'capsaicin' - the source of the heat in hot chili peppers. HHOH H HHH H -C-C-C-C-C%3DC-C-CH3 T T T T H HHH -C-N-C-C- H CH3 a. b. H. H. C- H3C-O C-C- C-C C. d.arrow_forwardWrite the common (not systematic) name of each organic molecule. Hint: your answer should have more than one word. structure O || CH3 CH₂ C-CH₂-CH₂ - CH3 || CH3CH₂CH₂ C-CH3 || CH3 -C- CH3 name 0 0 0 Xarrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
