
Concept explainers
(a)
Interpretation:
Whether the product of the given step can eliminate a leaving group to form a different compound than the reactant is to be predicted. The product for the given nucleophilic elimination step with appropriate curved arrows is to be drawn.
Concept introduction:
In the nucleophilic elimination step, the more electronegative atom bears full or partial negative charge. This is an electron rich atom, and the less electronegative atom is relatively electron poor. The curved arrow drawn from the lone pair of electron rich atom points to the bonding region between the more electronegative atom and less electronegative atom representing the electron flow from the electron rich site to the electron poor site. The second curved arrow is drawn to represent the breaking of the bond between the less electronegative atom and leaving group to avoid exceeding an octet on the less electronegative atom.
Answer to Problem 7.27P
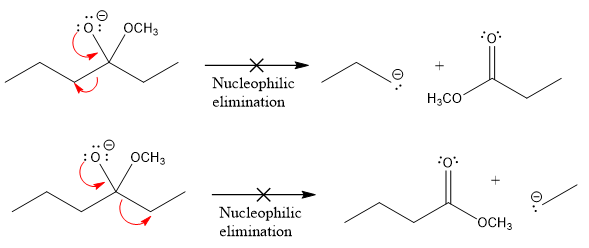
Products formed after the elimination of the leaving group are not the same as the reactant. Product formed in the nucleophilic elimination step with appropriate curved arrows is drawn as:
Explanation of Solution
Product for the given nucleophilic addition step is:
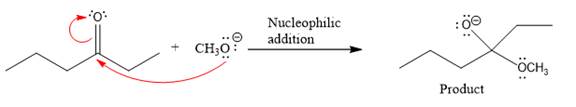
In the given product, there are two possible groups that can leave to form two different products.
In the first nucleophilic elimination step, the oxygen atom with negative charge is an electron rich site, and the carbon bonded to it is an electron poor site. The curved arrow mechanism for this given nucleophilic elimination step forming the respective product is:
The first curved arrow is drawn from the lone pair of negatively charged oxygen to the mid of
The respective product formed is different from the reactant in the given nucleophilic addition step. The X sign on the arrow represents that this nucleophilic elimination is unfeasible as
In the second nucleophilic elimination step, the oxygen atom with negative charge is the electron rich site, and the carbon bonded to it is the electron poor site. The curved arrow mechanism for this given nucleophilic elimination step forming the respective product is:
The first curve arrow is drawn from the lone pair of negatively charged oxygen to the mid of
The respective product formed is different from the reactant in the given nucleophilic addition step. The X sign on the arrow represents that this nucleophilic elimination is unfeasible as
Products formed in the elimination steps are different from the reactant in the given nucleophilic addition step.
(b)
Interpretation:
Whether the product of the given step can eliminate a leaving group to form a different compound than the reactant is to be predicted. The product for the given nucleophilic elimination step with appropriate curved arrows is to be drawn.
Concept introduction:
In the nucleophilic elimination step, the more electronegative atom bears full negative charge or partial negative charge. This is the electron rich atom and the less electronegative atom is relatively electron poor. The curved arrow drawn from the lone pair of electron rich atom points to the bonding region between the more electronegative atom and less electronegative atom representing the electron flow from the electron rich site to the electron poor site. The second curved arrow is drawn to represent the breaking of bond between the less electronegative atom and leaving group to avoid exceeding an octet on the less electronegative atom.
Answer to Problem 7.27P
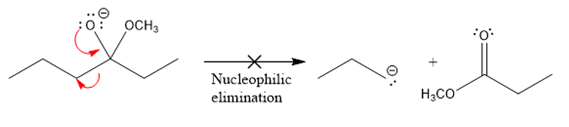
The product formed after the elimination of the leaving group is not the same as the reactant. Product formed in the nucleophilic elimination step with an appropriate curved arrow is drawn as:
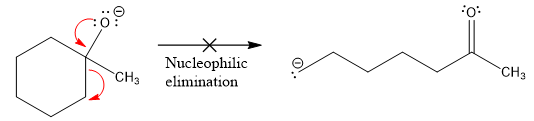
Explanation of Solution
Product for the given nucleophilic addition step is:
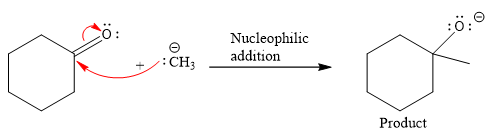
In the nucleophilic elimination step, the oxygen atom with negative charge is an electron rich site, and the carbon bonded to it is an electron poor site. The curved arrow mechanism for this given nucleophilic elimination step forming the respective product is:

The first curved arrow is drawn from the lone pair of negatively charged oxygen to the mid of
The respective product formed is different from the reactant in the given nucleophilic addition step. The X sign on the arrow represents this nucleophilic elimination is unfeasible as
Product formed in the elimination step is different from the reactant in the given nucleophilic addition step.
(c)
Interpretation:
The product of the given step can eliminate a leaving group to form different compound than reactant is to be predicted. The product for the given nucleophilic elimination step with appropriate curved arrows is to be drawn.
Concept introduction:
In nucleophilic elimination step, the more electronegative atom bears full negative charge or partial negative charge. This is the electron rich atom and the less electronegative atom is relatively electron poor. The curved arrow drawn from the lone pair of electron rich atom points to the bonding region between the more electronegative atom and less electronegative atom representing the electron flow from electron rich site to electron poor site. The second curved arrow drawn to represent the breaking of bond between the less electronegative atom and leaving group to avoid exceeding an octet on the less electronegative atom.
Answer to Problem 7.27P
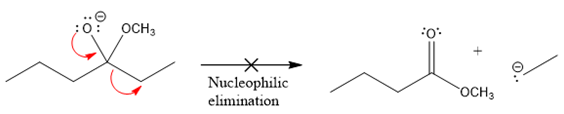
The products formed after the elimination of the leaving group are not the same as the reactant. Product formed in the nucleophilic elimination step with appropriate curved arrow is drawn as:
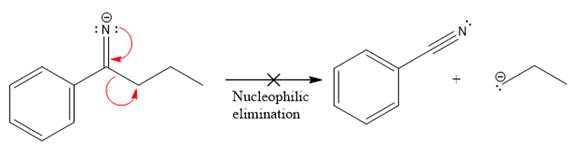
Explanation of Solution
Product for the given nucleophilic addition step is:
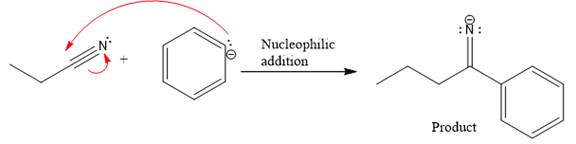
In the nucleophilic elimination step, the nitrogen atom with negative charge is electron rich site, and the carbon bonded to it is electron poor site. The curved arrow mechanism for this given nucleophilic elimination step forming the respective product is:

The first curved arrow is drawn from the lone pair of negatively charged nitrogen to the mid of
The respective product formed is different from the reactant in the given nucleophilic addition step. The X sign on the arrow represents this nucleophilic elimination is unfeasible as
Product formed in the elimination step is different from the reactant in the given nucleophilic addition step.
(d)
Interpretation:
Whether the product of the given step can eliminate a leaving group to form different compound than reactant is to be predicted. The product for the given nucleophilic elimination step with appropriate curved arrows is to be drawn.
Concept introduction:
In nucleophilic elimination step, the more electronegative atom bears full negative charge or partial negative charge. This is the electron rich atom and the less electronegative atom is relatively electron poor. The curved arrow drawn from the lone pair of electron rich atom points to the bonding region between the more electronegative atom and less electronegative atom representing the electron flow from electron rich site to electron poor site. The second curved arrow is drawn to represent the breaking of bond between the less electronegative atom and leaving group to avoid exceeding an octet on the less electronegative atom.
Answer to Problem 7.27P
Products formed after the elimination of the leaving group are not the same as the reactant. Product formed in the nucleophilic elimination step with appropriate curved arrow is drawn as:
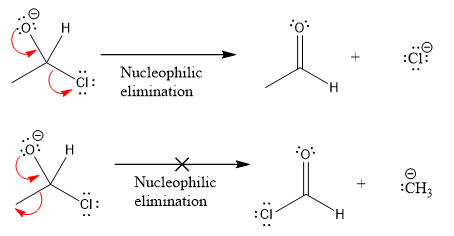
Explanation of Solution
Product for the given nucleophilic addition step is:
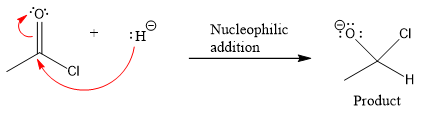
In the first nucleophilic elimination step, the oxygen atom with negative charge is electron rich site, and the chlorine atom is a good leaving group. The curved arrow mechanism for this given nucleophilic elimination step forming the respective product is:
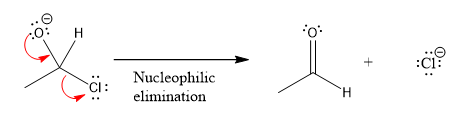
The first curved arrow is drawn from the lone pair of negatively charged oxygen to the mid of
The respective product formed is different from the reactant in the given nucleophilic addition step.
In the second nucleophilic elimination step, the oxygen atom with negative charge is electron rich site and the carbon bonded to it is electron poor site. The curved arrow mechanism for this given nucleophilic elimination step forming the respective product is:
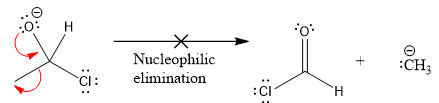
The first curved arrow is drawn from the lone pair of negatively charged oxygen to the mid of
The respective product formed is different from the reactant in the given nucleophilic addition step. The X sign on the arrow represents this nucleophilic elimination is unfeasible as
Products formed in the elimination steps are different from the reactant in the given nucleophilic addition step.
(e)
Interpretation:
Whether the product of the given step can eliminate a leaving group to form different compound than reactant is to be predicted. The product for the given nucleophilic elimination step with appropriate curved arrows is to be drawn.
Concept introduction:
In nucleophilic elimination step, the more electronegative atom bears full negative charge or partial negative charge. This is the electron rich atom and the less electronegative atom is relatively electron poor. The curved arrow is drawn from the lone pair of electron rich atom points to the bonding region between the more electronegative atom and less electronegative atom representing the electron flow from electron rich site to electron poor site. The second curved arrow is drawn to represent the breaking of bond between the less electronegative atom and leaving group to avoid exceeding an octet on the less electronegative atom.
Answer to Problem 7.27P
Products formed after the elimination of the leaving group are not same as the reactant. Product formed in the nucleophilic elimination step with appropriate curved arrow is drawn as:
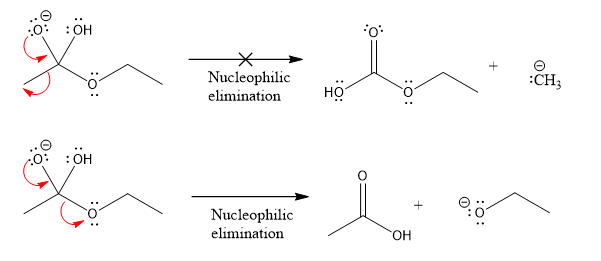
Explanation of Solution
Product for the given nucleophilic addition step is:
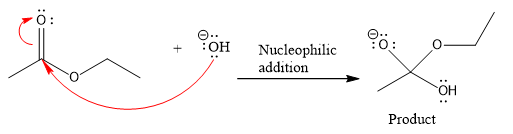
In the given product, there are two possible groups that can leave to form two different products.
In the first nucleophilic elimination step, the oxygen atom with negative charge is electron rich site, and the carbon bonded to it is electron poor site. The curved arrow mechanism for this given nucleophilic elimination step forming the respective product is:
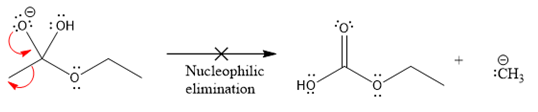
The first curved arrow is drawn from the lone pair of negatively charged oxygen to the mid of
The respective product formed is different from the reactant in the given nucleophilic addition step. The X sign on the arrow represents this nucleophilic elimination is unfeasible as
In the second nucleophilic elimination step, the oxygen atom with negative charge is electron rich site and
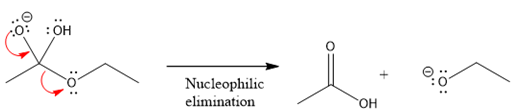
The first curved arrow is drawn from the lone pair of negatively charged oxygen to the mid of
The respective product formed is different from the reactant in the given nucleophilic addition step.
Products formed in the elimination steps are different from the reactant in the given nucleophilic addition step.
(f)
Interpretation:
Whether the product of the given step can eliminate a leaving group to form different compound than reactant is to be predicted. The product for the given nucleophilic elimination step with appropriate curved arrows is to be drawn.
Concept introduction:
In nucleophilic elimination step, the more electronegative atom bears full negative charge or partial negative charge. This is the electron rich atom and the less electronegative atom is relatively electron poor. The curved arrow drawn from the lone pair of electron rich atom points to bonding region between the more electronegative atom and less electronegative atom representing the electron flow from electron rich site to electron poor site. The second curved arrow is drawn to represent the breaking of bond between the less electronegative atom and leaving group to avoid exceeding an octet on the less electronegative atom.
Answer to Problem 7.27P
Products formed after the elimination of the leaving group are not same as the reactant. Product formed in the nucleophilic elimination step with appropriate curved arrow is drawn as:
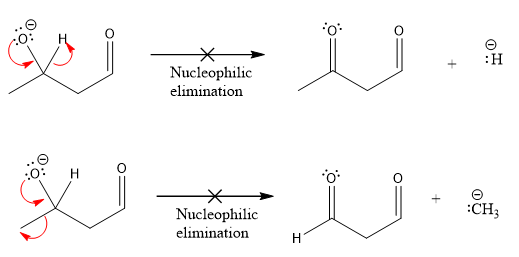
Explanation of Solution
Product for the given nucleophilic addition step is:

In the given product, there are two possible groups that can leave to form two different products.
In the first nucleophilic elimination step, the oxygen atom with negative charge is electron rich site and the carbon bonded to it is electron poor site. The curved arrow mechanism for this given nucleophilic elimination step forming the respective product is:

The first curved arrow is drawn from the lone pair of negatively charged oxygen to the mid of
The respective product formed is different from the reactant in the given nucleophilic addition step. The X sign on the arrow represents this nucleophilic elimination is unfeasible as
The second nucleophilic elimination step, the oxygen atom with negative charge is electron rich site and the carbon bonded to it is electron poor site. The curved arrow mechanism for this given nucleophilic elimination step forming the respective product is:

The first curved arrow is drawn from the lone pair of negatively charged oxygen to the mid of
The respective product formed is different from the reactant in the given nucleophilic addition step. The X sign on the arrow represents this nucleophilic elimination is unfeasible as
Products formed in the elimination steps are different from the reactant in the given nucleophilic addition step.
Want to see more full solutions like this?
Chapter 7 Solutions
Organic Chemistry: Principles and Mechanisms (Second Edition)
- Explain how you can tell from the energy diagram that the reaction with the catalyst in Fig. 8.4 isfaster than the reaction without the catalyst.arrow_forwardIdentify the electrophile and the nucleophile in each of the following reaction steps. Then draw curved arrows to illustrate the bond-making and bond-breaking processes.arrow_forward7. (Chapters 6 and 8) Within the following set, which is more stable, and why? CH3 CH3 H3C- -C=CH- CH2 H2C=Ć- -CH CH3 8. (Chapter 12) What type of instability will an intermediate need to address following the reaction of a nucleophile/base that has a negative charge with a pi bond that has uneven electron distribution between atoms with different electronegativities (C=O)? 9. (Chapter 9) Circle the carbon that will be unstable in the intermediate of the following reaction. Then, state the reason for your choice, and also indicate what type of instability it will be. H,C-CH,- C ECH with NaNH2 10. (Chapters 12 and 13) What are three sources used to provide electrons to an electron-deficient carbon with a leaving group? 1. 2. 3.arrow_forward
- Which of the following is the rule which states that the more substituted product is the major product in an elimination reaction? O 1) Boyle's Law O 2) Markovnikov's Rule 3) Zaitsev's Rule O 4) LeChatlier's Principlearrow_forward6). What do you think would be the better nucleophile? Cu Organocopper reagent Cu Organocuprate reagentarrow_forward1. What is the nucleophile in this reaction? 2. Draw the energy profile for this reaction.arrow_forward
- Complete the following stepwise reaction mechanism problems based on the reaction conditions given: Draw the stepwise mechanism.arrow_forwardDraw the major organic product of the bimolecular substitution and use curved-arrow notation to draw the mechanism. Be sure to draw any non-bonding electrons. Step 1: Draw curved arrows. Step 2: Draw the product. +NaI N N DMSOarrow_forwardDraw a complete, step-wise, curved arrow mechanism for each reaction shown below. You don't need to worry about stereochemistry for these problems. It may help if you take the following steps. 1) Find the nucleophile and the electrophile. 2) Determine the major functional group present in the nucleophile and electrophile. 3) Determine the type of reaction this particular nucleophile/electrophile pair is likely to participate in 4) Draw the mechanism that corresponds with this reaction type. a) OH cat. H2SO4 HO Cl2 b) :OHarrow_forward
- 1. Copy and complete the following table by indicating whether each of the following scenarios would either INCREASE or DECREASE the rate of reaction. Scenario Putting reaction vessel in the fridge Decreasing temperature Removing source of heat energyN Lowering temperature Putting reaction mixture in a hot oven Rate of Reactionarrow_forwardPlease draw the missing product and the arrow pushing mechanism. Please identify all charged atoms and intermediates for each steparrow_forwardOn a scrap piece of paper, draw the curved arrow mechanism for the following reaction. Once you have determined the major product, draw it in the space below. H -OH NaOH(trace)arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
