
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN: 9780618974122
Author: Andrei Straumanis
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 6, Problem 22E
Double bonds do not rotate freely under normal conditions. The change from Z to E requires areaction. This can occur in the presence of a catalyst or with the addition of a large amount ofenergy (e.g., at high temperature).
One such reaction is diagramed below:
(1) Add enough potential energy to break the double bond
(2) free rotation occurs at high energy transition state, then
(3) reforming the double bond as a mixture of Z and E.
Draw E-2-butene in one box and Z-2-butene in the other box, and explain your reasoning.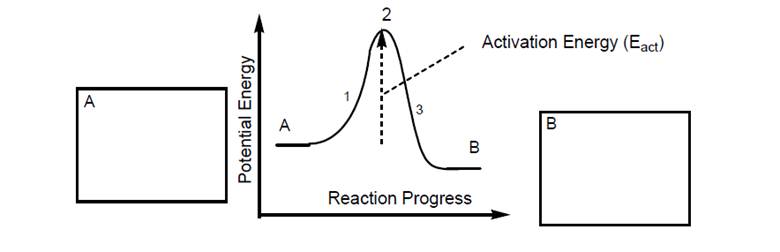
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
What is the total strain energy of 2,2-dimethylpropane in an eclipsed
conformation? Given: For CH3 eclipsed to H (both attached to adjacent
C's), the total strain energy is 6 kJ/mol.
Select one:
A. 12 kJ
В. 18 kJ
С. 21 kJ
D. 11.4 kj
5. For the pair of compounds circle the compound which is more stable (you may find it
helpful to draw out the chair conformation)
4. Zingiberene, a terpene found in ginger, has the following structure. Classify the rt?bonds in
zingiberene as conjugated, cumulated or isolated.
Chapter 6 Solutions
Organic Chemistry: A Guided Inquiry
Ch. 6 - Prob. 1CTQCh. 6 - Prob. 2CTQCh. 6 - Prob. 4CTQCh. 6 - Prob. 5CTQCh. 6 - Complete this graph of relative potential energy...Ch. 6 - Prob. 7CTQCh. 6 - Prob. 8CTQCh. 6 - Prob. 9CTQCh. 6 - Consider the Newman projection below. a. Draw a...Ch. 6 - Draw a Newman projection showing the lowest P.E....
Ch. 6 - Prob. 12CTQCh. 6 - Prob. 13CTQCh. 6 - In skeletal representations the hydrogens are not...Ch. 6 - Prob. 15CTQCh. 6 - Prob. 16CTQCh. 6 - Prob. 17CTQCh. 6 - Prob. 19CTQCh. 6 - Prob. 20CTQCh. 6 - Prob. 21CTQCh. 6 - Prob. 22CTQCh. 6 - Prob. 23CTQCh. 6 - Draw a constitutional isomer of pentane,...Ch. 6 - How many H’s are lost from the molecular formula...Ch. 6 - How many ifs are lost from the molecular formula...Ch. 6 - Prob. 27CTQCh. 6 - What is the degree of unsaturation for the example...Ch. 6 - Without counting hydrogens, determine which one of...Ch. 6 - Determine the degree of unsaturation (and draw a...Ch. 6 - a model of each molecule shown above: Is the...Ch. 6 - Prob. 32CTQCh. 6 - Prob. 33CTQCh. 6 - Label each double bond E, Z, or neither. (It may...Ch. 6 - Prob. 35CTQCh. 6 - Prob. 36CTQCh. 6 - Indicate the relationship between each pair....Ch. 6 - Prob. 38CTQCh. 6 - Prob. 1ECh. 6 - Prob. 2ECh. 6 - Using your model of butane (CH3CH2CH2CH3) ,...Ch. 6 - Consider the molecule 1-bromo-2-methylbutane. C3...Ch. 6 - Prob. 5ECh. 6 - Prob. 8ECh. 6 - Prob. 9ECh. 6 - Prob. 10ECh. 6 - Prob. 11ECh. 6 - Prob. 12ECh. 6 - Prob. 13ECh. 6 - Prob. 15ECh. 6 - Prob. 16ECh. 6 - Prob. 17ECh. 6 - Prob. 18ECh. 6 - Prob. 19ECh. 6 - Prob. 20ECh. 6 - Prob. 21ECh. 6 - Double bonds do not rotate freely under normal...Ch. 6 - up an example (not appearing in this ChemActivity)...Ch. 6 - Prob. 24ECh. 6 - Prob. 25E
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- (a) Using Newman projections, draw all staggered and eclipsedconformations that result from rotation around the bond highlighted in red in each molecule; (b) draw a graph of energy versus dihedral angle for rotation around this bond.arrow_forwardConsider the substituted cyclohexane shown in the ball-and-stick model. (See attached) Draw the second possible conformation in the chair form, and classifyit as more stable or less stable than the conformation shown in thethree-dimensional model ?arrow_forwardFor the cyclohexane molecules a and b draw the most stable chair formarrow_forward
- Write a structure ( Chair conformation )arrow_forwardIn this molecule’s other chair conformation, how many (non H) axial positions are there?arrow_forwarda. Draw the lowest energy conformation for the compound shown in the image. b. Draw the complete structural formula of the following compound: cis-1-bromo-3-chlorocyclohexane.arrow_forward
- What is the most stable chair conformation of the attached molecule?arrow_forwardDraw the most stable conformationarrow_forward4) Draw an alkene (minimum of 4 carbons) that is in the E conformation and a different alkene (also 4 carbon minimum) that is in the Z conformation. Provide an explanation as to what makes it E or Z.arrow_forward
- 1E.) Which of the following two conformers is lower in energy? Both conformers are of equal enery O A Conformer A is lower in energy. B Conformer B is lower in energy. It cannot be determinedarrow_forward3. Draw all possible conformations for 2-methylbutane. Label all unfavorable interactions. Which conformation is most stable? Explain.arrow_forwardThe correct statement(s) for the following addition reactions is (are) H3C 1. BD3 THF i) M and N 2. H2O2, OH CH3 H3C CH3 1. BD3 THF ii) O and P 2. H2О, ОН- H [0] and [P] are identical molecules [M] and [N] are enantiomers First reaction yields racemic mixture; whereas second reaction yields meso isomers Total three fractions obtained, when we distilled product mixture of both the reactionsarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Nomenclature: Crash Course Chemistry #44; Author: CrashCourse;https://www.youtube.com/watch?v=U7wavimfNFE;License: Standard YouTube License, CC-BY