
Concept explainers
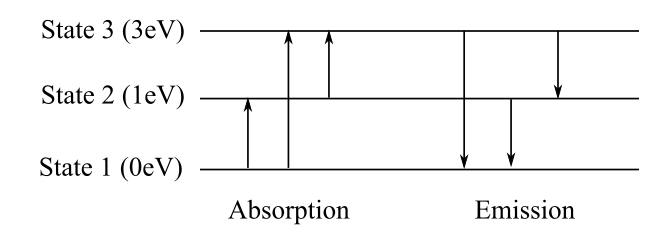
The diagram showing all possible transitions between the levels of an imaginary atom, having energy
Answer to Problem 41Q
Solution:
The diagram representing all possible transitions is shown below. The wavelengths for the energy differences
Explanation of Solution
Given data:
The energy values of the given atomic levels are
Formula used:
Planck’s quantum theory relates the energy difference between the transition levels to the frequency of the released
Or,
Here,
Explanation:
The given atom has only three energy levels. Let, state 1 represents the level with energy
Write the expression relating the energy and wavelength of the photon:
Here,
Estimate the energy difference of the levels.
The difference between first two energy states is,
Similarly,
And,
Rewrite the above expression for energy difference for wavelength.
Substitute
Substitute
All the possible transitions are shown in the following diagram.

The absorption or emission transition of visible lights are between the states 2 and 3 and 1 and 3 as the wavelength for these transitions lie in the visible region.
Conclusion:
The energy level diagram is shown above. The values of energy of the photons are
Want to see more full solutions like this?
Chapter 5 Solutions
Universe
- Iron has a magnetic moment of 2.22 Bohr magnetons per atom and a density of 7.87-103 kg.m-3. Calculate the expected magnetization of iron at 0 K and describe any assumptions that you have made. How would you expect this magnetization of iron to vary as temperature is increased. How does the number of Bohr magnetons per atom change from 0 K to 300 K. Why does a piece of iron typically not exhibit high magnetization at room temperature (unless it has been "magnetized")?arrow_forwardFor the purpose of this exercise, we consider the Earth as a blackbody at a temperature of 300K. a. Assuming that it is spherical with a radius equals to 6370 km, calculate the total amount energy emitted by the Earth (Hint: The total amount of energy emitted by a surface = amount of energy emitted per unit area x area of the surface). b. What wavelength range would you recommend to measure radiation emitted by the Earth using a satellite mounted sensor?arrow_forwardCalculate the energy of a 800 nm photon. b. Find the wavelength and frequency of a 80-MeV photon. Given that the maximum wavelength for photoelectric emission in tungsten is 230 nm, what wavelength of light must be used to eject electrons with a maximum energy of 1.6 eV?arrow_forward
- A perfect black body has its surface temperature 27 cº Determine : Maximum radiation wavelength? Black body radiation intensity? The rate of energy released from 2m² Tungsten wire had its radiating surface area 8mm² and its temperature 2100K, considering that the wire is an ideal black body, Calculate the energy that the wire radiates in 10 minutes. Suppose the surface temperature of the Sun were about 12,000K, rather than 6000K. a. How much more thermal radiation would the Sun emit? b. What would happen to the Sun's wavelength of peak emission? c. Do you think it would still be possible to have life on Earth? Explain /A The energy radiated by a black body at 2300K is found to have the maximum at a wavelength 1260 nm, its emissive power being 8000W/m2. When the body is cooled to a temperature T K, the emissive power is found to decrease to 500W/m2. Find : (i) the temperature T k (ii) the wave length at which intensity of emission in maximum at the Te / Black body becomes yellow with λ…arrow_forwardThe Pfund series in the hydrogen spectrum corresponds to transitions that have a final state of m=5. A. What are the wavelengths of the first three lines in this series? Express your answers in micrometers to three significant figures. Enter your answers in descending order separated by commas. B. What part of the electromagnetic spectrum are these lines in?arrow_forwardThe first three energy levels of the fictitious element X are as shown.a. What wavelengths are observed in the absorption spectrum of element X? Give your answers in nm.b. State whether each of your wavelengths in part a corresponds to ultraviolet, visible, or infrared light.c. An electron with a speed of 1.4 × 106 m/s collides with an atom of element X. Shortly afterward, the atom emits a 1240 nm photon. What was the electron’s speed after the collision? Assume that, because the atom is so much more massive than the electron, the recoil of the atom is negligible.arrow_forward
- The figure at the right shows transitions between three states of a molecule (labeled E1, E2, and E3) that are observed through the emission and absorption of photons in a pair of experiments, A and B.1. Which transition is associated with the emission of the photon of the longest wavelength?a b c d e2. Which transition is associated with the absorption of the photon of the shortest wavelength?a b c d e 3. If a cold gas of these molecules was in the atmosphere of a star that was emitting a continuous spectrum, you would expect to see some dark lines across the continuous spectrum from that star. This is because the molecules in the atmosphere of the star absorb some of the light emitted by the star. Which frequencies would you expect to be significantly blocked by passing through the cold gas?a. (E2 - E1)/hb. (E3 E1)/hc. (E3 E2)/hd. None of them.a b c darrow_forwardAnswer the following A. A comet has just passed the Earth and its peak emission is observed at 15000 nm. Determine in which region of the electromagnetic spectrum (e.g. X-ray, infrared, visible, ultraviolet, ...) the peak emission wavelength resides. What is the temperature of the comet? B. Within the Solar System, a convenient unit of measurement is the Earth-Sun distance, called an astronomical unit (AU). For bigger distances, we use the light year (LY), the distance that light travels in one year. We can expand our lingo to include other measures of distance, for example, light days, light minutes, and light hours. Starting with the values you can look up in the Appendices for the speed of light and the astronomical unit, calculate how many “light minutes” there are in 1 AU. C. What is the observable universe? How big is it?arrow_forwardAs shown the energy-level diagram of Element X.a. What is the ionization energy of Element X?b. An atom in the ground state absorbs a photon, then emits a photon with a wavelength of 1240 nm. What conclusion can you draw about the energy of the photon that was absorbed?c. An atom in the ground state has a collision with an electron, then emits a photon with a wavelength of 1240 nm. What conclusion can you draw about the initial kinetic energy of the electron?arrow_forward
- 1. What is the wavelength of a radio photon from an AM radio station that broadcasts at 1470 kilohertz? Express your answer to three significant figures and include the appropriate units. 2. What is the energy of a radio photon from the same station? Express your answer to three significant figures and include the appropriate units. For question 1 I am not sure how to solve it because the frequency is in kilohertz and I don't know how to convert it into something that I can use. For the speed of light in this question, would it be best to use 3 x 10^8 m/s or 3 x 10^5 km/s? For question 2 I have the formula to use, I'm just uncertain of how to use it.arrow_forwardConsider an electron in a hydrogen atom that undergoes a transition from n = 3 to n = 7. a. Is the atom undergoing absorption or emission? b. What is the change in energy of the atom? c. What is the wavelength (in nm) of the photon absorbed or emitted? d. What is the frequency of the photon absorbed or emitted?arrow_forwardIn the energy-level diagram shown for a gas, the wavelength of the radiation associated with transition A is 600 nm , and that associated with transition B is 300 nm. a. Determine the energy of a photon associated with transition A. eV. b. Determine the A of the radiation associated with transition C. nm. c. What will happen to a beam of white light (400 to 600 nm) that is sent through this gaseous element?arrow_forward
 Modern PhysicsPhysicsISBN:9781111794378Author:Raymond A. Serway, Clement J. Moses, Curt A. MoyerPublisher:Cengage Learning
Modern PhysicsPhysicsISBN:9781111794378Author:Raymond A. Serway, Clement J. Moses, Curt A. MoyerPublisher:Cengage Learning
