
Organic Chemistry: Structure and Function
8th Edition
ISBN: 9781319079451
Author: K. Peter C. Vollhardt, Neil E. Schore
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 4, Problem 57P
Interpretation Introduction
Interpretation:Cycloalkane that has the greatest ring strain should be chosen.
Concept introduction:Ring strainis a kind of instability that results when bonds deviate from ideal bond-angle. The cyclic system has different amount of strain as illustrated below:
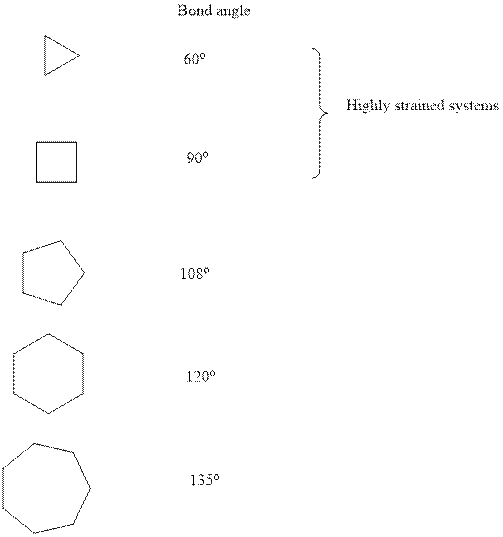
It is evident that three and four membered small ring systems have considerable degree of angular and ring strain that leads to their instability.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
1. Draw the structure of each of the following cycloalkanes a. 1-Bromo-2-methylcyclobutaneb. 1,2-Dibromo-3-methylcyclohexanec. Iodocyclopropane2. How does the general formula of a cycloalkane compare to that of an alkane?
2. Determine whether cis-trans isomerism is possible for each of the following
cycloalkanes. If so, then draw structural formulas for the cis and trans isomers..
a. Methylcyclohexane
b. 1,1-Dimethylcyclohexane
c. 1,3-Dimethylcyclobutane
d. 1-Ethyl-2-methylcyclobutane
Which of the following correctly ranks the
?cycloalkanes in order of increasing ring strain per methylene
اختر أحد الخيارات
a. cyclopropane < cyclobutane < cyclohexane <
cycloheptane
b. cyclohexane < cyclopentane < cyclobutane <
cyclopropane
c. cyclopentane < cyclopropane < cyclobutane <
cyclohexane
d. cyclopentane < cyclobutane < cyclopentane <
cyclopropane
Chapter 4 Solutions
Organic Chemistry: Structure and Function
Ch. 4.1 - Prob. 4.1ECh. 4.1 - Prob. 4.3TIYCh. 4.2 - Prob. 4.4ECh. 4.2 - Prob. 4.6TIYCh. 4.3 - Prob. 4.7ECh. 4.4 - Prob. 4.8ECh. 4.4 - Prob. 4.10TIYCh. 4.4 - Prob. 4.11ECh. 4.4 - Prob. 4.12ECh. 4.4 - Prob. 4.14TIY
Ch. 4.6 - Prob. 4.15ECh. 4.7 - Prob. 4.16ECh. 4.7 - Prob. 4.17ECh. 4.7 - Prob. 4.18ECh. 4 - Prob. 21PCh. 4 - Prob. 22PCh. 4 - Prob. 23PCh. 4 - Prob. 24PCh. 4 - Prob. 25PCh. 4 - Prob. 26PCh. 4 - Prob. 27PCh. 4 - Prob. 28PCh. 4 - Prob. 29PCh. 4 - Prob. 30PCh. 4 - Prob. 31PCh. 4 - Prob. 32PCh. 4 - Prob. 33PCh. 4 - Prob. 34PCh. 4 - Prob. 35PCh. 4 - Prob. 36PCh. 4 - Prob. 37PCh. 4 - Prob. 38PCh. 4 - Prob. 39PCh. 4 - Prob. 40PCh. 4 - Prob. 41PCh. 4 - Prob. 42PCh. 4 - Prob. 43PCh. 4 - Prob. 44PCh. 4 - Prob. 45PCh. 4 - Prob. 46PCh. 4 - Prob. 47PCh. 4 - Prob. 48PCh. 4 - Prob. 49PCh. 4 - Prob. 50PCh. 4 - Prob. 51PCh. 4 - Prob. 52PCh. 4 - Prob. 53PCh. 4 - Prob. 54PCh. 4 - Prob. 55PCh. 4 - Prob. 56PCh. 4 - Prob. 57PCh. 4 - Prob. 58PCh. 4 - Prob. 59PCh. 4 - Prob. 60P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- How does the structure of a cycloalkane differ from that of a straight-chain or branched-chain alkane?arrow_forwardDetermine whether cis-trans isomerism is probable for the following cycloalkanes. If it is possible, draw structural formulas for the cis and trans isomers. a. 1-ethyl-1-methylcyclopentane b. ethylcyclohexane c. 1,4-diethylcyclohexane d. 1,1-dimethylcyclooctane e. methylcycloheptanearrow_forward1. Identify the SYSTEMATIC name of the aliphatic hydrocarbon a. 1-pentenyicyclopentane b. 1-cyclopentylpent-2-yne c. 1-pentenecyclopent-2-yne d. 1-pentenyicyclopentane 2. Identify the SYSTEMATIC name of the aliphatic hydrocarbon a. Trans-1,2-propylcyclopropane b. Trans-1,2-diisopropylcyclopropane c. Cis-1,2-propylcyclopropane d. Cis-1,2-diisopropylcyclopropane 3. Identify the SYSTEMATIC name of the aliphatic hydrocarbon 3,10-dimethyl-2-decacen-6-yne 3,10-dimethyl-2-dodocen-6-yne 3,10-dimethyl-10-decacen-6-yne d. 3,10-dimethyl-10-dodocen-6-yne a. b. C. 4. Identify which type of isomer the following structures represent HO a. Skeletal Isomer b. E/Z isomer c. Cis/Trans isomer OH OH d. Positional isomer 5. Identify which type of isomer the following structures represent a. Skeletal Isomer Br b. E/Z isomer c Cis/Trans isomer Br d. Positional isomerarrow_forward
- Which of the following names are correct and which areincorrect? If incorrect, write the correct name.a. 2,3-Dibromocyclobutane c. 1,2-Dimethylcyclopropaneb. 1,4-Diethylcyclobutane d. 4,5,6-Trichlorocyclohexanearrow_forward1. What is the alkane product from the reaction of C7H13COONa and NaOH? a. pentane b. hexane c. butane d. heptane 2. What is the resulting alkane if we have C5H11F and a C5H11F as reactants in Wurtz synthesis? a. hexane b. octane c. nonane d. decane 3.What is the resulting alkane if we have 2C2H5Cl as reactants in Wurtz Synthesis reaction? a. ethane b. butane c. hexane d. octanearrow_forwardWhich among the following compounds has the most electronegative carbon in structure? a. benzaldehyde b. benzene c. cyclobutane d. butyne The radical substitution reaction is primarily observed in the following reactions. Which reaction is it? a. Oxidation of but-2-ene b. Boiling of hex-3-ene c. Chlorination of butane d. Combustion of methane Which among the following statements is FALSE about alkanes and cycloalkanes? a. all alkanes produce CO2 and water vapor upon complete combustion b. alkanes undergo substitution reactions c. the empirical formula of all alkanes is CnH2n+2 d. cyclic alkanes exhibit bond strain in the sp#-sp# sigma bondsarrow_forward
- Which of the following statements regarding cycloalkanes is wrong? a. The least strained form of any unsubstituted cycloalkane is the boat conformation of cyclohexane b. The planar form of any cycloalkane with a ring larger than cyclopropane will not be the most stable conformation c. Cyclopentane is nonplanar to avoid the torsional strain between adjacent C-H bonds. d. A di-substituted cycloalkane can have cis-trans isomers.arrow_forward7. C=C. A hydrocarbon that contains a -C=C- or the above group. Oa. addition reaction Ob. aliphatic compound C. alkene Od. alkyne e. aromatic hydrocarbon f. hydration g. hydrogenation h. monomer i. phenyl group j. polycyclic aromatic hydrocarbon Ok. polymer O1. unsaturated hydrocarbonarrow_forwardWrite skeletal formulas for the following alkanes and cycloalkanes. Use solid and broken lines to indicate stereochemistry. a.3-ethyl-2,4,5-trimethyloctane b.4-(1-methylethyl)octane c.5-butyl-2,2-dimethylnonane d.trans-1,3-dimethylcyclopentane e.cis-1,2-diethylcyclobutanearrow_forward
- How many of the 18 Cg alkane constitutional isomers are named using the following base-chain names? a. Octane b. Heptane с. Нехаne d. Pentane How many of the nine C7 alkane constitutional isomers are named using the following base-chain names? а. Неptane b. Нехane c. Pentane d. Butanearrow_forward1. Name and draw the isomers form from the given molecular formula. a. C₂H16 b. C4H,Br₂ 2. Draw the structure of each of the following cycloalkanes a. 1-Bromo-2-methylcyclobutane b. 1,2-Dibromo-3-methylcyclohexanearrow_forwardUsing the IUPAC system, name the following alkanes: A. CH3-CH2-CH-CH2-CH3 | CH3 B. CH3-CH-CH2-CH2-CH3 | CH2 | CH3 CH3 CH3 | | C. CH3-C-CH2-C-CH3 | | CH3 CH3arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning  Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning


Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning