
Concept explainers
Interpretation:
The structural formula for each of the given compounds is to be drawn.
Concept introduction:
The systematic naming of organic compound is given by IUPAC. The naming of organic compound is done in such a way that the structure of organic compound is correctly interpreted from the name.
Rules for writing the structural formula from IUPAC are:
First identify the word root for the given compound.
The suffix used in the compound like –ane, ene, yne, ol, al and so on.
Identify the position, location, and number of the substituent bonded to the carbon chain.
Answer to Problem 28P
Solution:
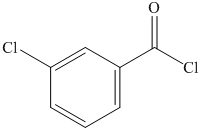
a) The structural formula of m-chlorobenzoyl chlorideis shown below.
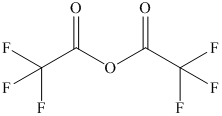
b) The structural formula of trifluoroacetic anhydride is shown below.
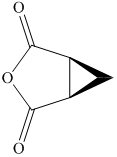
c) The structural formula of cis-
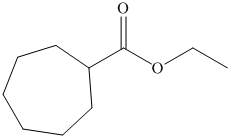
d) The structural formula of ethyl cycloheptanecarboxylateis shown below.
(e) The structural formula of
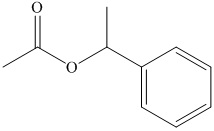
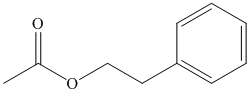
(f) The structural formula of
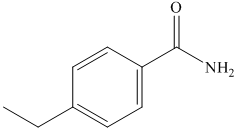
(g) The structural formula of p-ethylbenzamide is shown below.
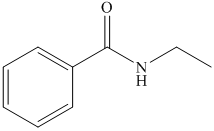
(h) The structural formula of N-ethylbenzamide is shown below.
(i) The structural formula of
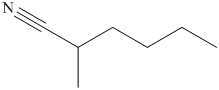
Explanation of Solution
a) m-Chlorobenzoyl chloride.
The name of compound suggests that chlorine group is present at meta position with respect to acyl group on benzene ring. The structure of compound is shown below.

b) Trifluoroacetic anhydride.
The name of the compound shows that anhydride group
group. The structure of compound is shown below.

c) cis-
The name of the compound indicates that two carboxylic groups are present on cyclopropane. Both carboxylic groups are present on the same plane. The structure of compound is shown below.

d) Ethyl cycloheptanecarboxylate
The name of the compound suggests that ester group is present in the structure. The cycloheptane ring is bonded to carbonyl carbon atom and ethyl group is bonded to oxygen atom. The structure of compound is shown below.

(e)
The name of the compound suggests that ester group is present in the structure. The methyl group is bonded to carbonyl carbon atom and ethyl group is bonded to oxygen atom. The structure of compound is shown below.

(f)
The name of the compound suggests that ester group is present in the structure. The methyl group is bonded to carbonyl carbon atom and ethyl group is bonded to oxygen atom. The second carbon atom is bonded to benzene ring. The structure of compound is shown below.

(g) p-Ethylbenzamide
The name of compound suggests that ethyl group is present at para position with respect to amide group on benzene ring. The structure of compound is shown below.

(h) N-Ethylbenzamide
The name of compound suggests that ethyl group is bonded to amide group which further bonded to benzene ring. The structure of compound is shown below.

(i)
The name of the compound indicates that it contains hydrocarbon chain of six carbon atoms. On the second carbon atom methyl group is present and on the first carbon atom nitrile group is present. The structure of compound is shown below.

Want to see more full solutions like this?
Chapter 20 Solutions
Organic Chemistry - Standalone book
- Predict the products formed when cyclohexanone reacts with the following reagents.phenylhydrazine and weak acidarrow_forwardWhat class of organic compound is formed upon the hydrolysis of benzoic acid anhydride? O carboxylic acid acid anhydride ester alcoholarrow_forwardThe following molecule belongs to a class of compounds called enediols. Each carbon of the double bond carries an —OH group: Draw structural formulas for the α-hydroxyketone and the α-hydroxyaldehyde with which this enediol is in equilibrium. α-hydroxyketone α-hydroxyaldehydearrow_forward
- Draw structures for the following compounds. ethylheptanoate methylpentanoate propyl-3-pentenoate methylpropanoate pentylethanoate ethylethanoate (ethyl-acetate) methylhexanoate propyloctanoate ethyl-5-hexynoate pentyl-5-heptenoatearrow_forwardWhat products are formed by acidic hydrolysis of the following compound? benzaldehyde and butan-1-d benzeie acid and butan-2-d 2-methylbutanoic acid and phenol benzoic acid and methyl ethyl ketonearrow_forwardWhat is the correct IUPAC name for the following molecule? ON-ethyl-N-methyl-2-propyl cyclohexanamine O N-ethyl-N-methyl-6-propyl cyclohexanamine IN ON-ethyl-N-methyl-1-propyl-2-cyclohexanamine ⒸN-cthyl-N-methyl-2-propyl-N-cyclohexanamine Previous Nextarrow_forward
- Draw the structure for the following compounds It organic 1: 2,2 diiodo-3-pentenoic acid 2: N,N-diethylpropanamide 3: 2-pentanone 4: phenoxy benzene 5: N,N-diethylpropanamidearrow_forwardWhat are your final product when 2-nitrobenzaldehyde is mixed with each of these: 4-cyanophenylhydrazine hydrochloride aminoguanidine bicarbonate 4-chlorophenylhydrazine hydrochloridearrow_forward17-67 Draw structural formulas for these compounds. (a) 1-Chloro-2-propanone (b) 3-Hydroxybutanal (c) 4-Hydroxy-4-methyl-2-pentanone (d) 3-Methyl-3-phenylbutanal (e) 1,3-Cyclohexanedione (f) 5-Hydroxyhexanalarrow_forward
- 17-72 The following molecule is an enediol; each carbon of the double bond carries an —OH group. Draw structural formulas for the hydroxyketone and the a-hydroxyaldehyde with which this enediol is in equilibrium.arrow_forwardShow the structures of the missing substance(s) in each of the following acid-base equilibria. a. CH3CH2NH2 + H2O ? + OH b. Diethylamine + H2Oarrow_forward
 Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning



