
Concept explainers
(a)
Interpretation: The full name along with the anomeric designation of the following compound should be determined:
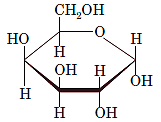
Concept Introduction: A common way of writing structural formula which represent the cyclic structure of monosaccharides is said to be the Haworth projection, which is best represented by chair conformations.
A convention used to represent a 3-D stereo formula in 2-D representation is said to be Fischer projections. In this projection, the vertical lines represent bonds below the plane of the paper and horizontal lines represents bonds above the plane of the paper.
(a)
Answer to Problem 21P
Explanation of Solution
Drawing the Fischer projection of given compound as:
For a D-sugar, the group attached to the bottom chiral carbon is present at the right-hand side whereas for L-sugar it is present at left-hand side.
A new carbon stereocenter created by the conversion of an open structure to a cyclic structure is said to be the anomeric carbon. In carbohydrates, when the hydroxy group on an anomeric carbon is present on the same side as that of the
The anomeric carbon of the given compound is shown by “*”:
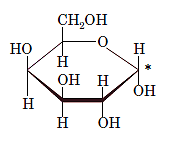
Since, in the given sugar the
Hence, the name of compound is
(b)
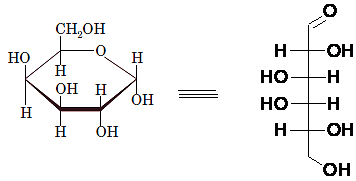
Interpretation: The full name along with the anomeric designation of the following compound should be determined:
Concept Introduction: A common way of writing structural formula which represent the cyclic structure of monosaccharides is said to be the Haworth projection, which is best represented by chair conformations.
A convention used to represent a 3-D stereo formula in 2-D representation is said to be Fischer projections. In this projection, the vertical lines represent bonds below the plane of the paper and horizontal lines represents bonds above the plane of the paper.
(b)
Answer to Problem 21P
Explanation of Solution
Drawing the Fischer projection of given compound as:
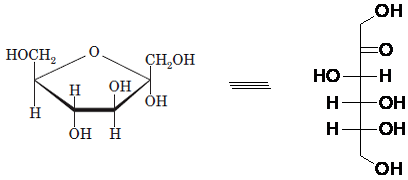
For a D-sugar, the
A new carbon stereocenter created by the conversion of an open structure to a cyclic structure is said to be the anomeric carbon. In carbohydrates, when the hydroxy group on an anomeric carbon is present on the same side as that of the
The anomeric carbon of the given compound is shown by “*”:
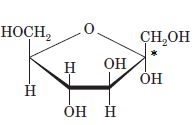
Since, in the given sugar the
Hence, the name of compound is
(c)
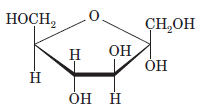
Interpretation: The full name along with the anomeric designation of the following compound should be determined:
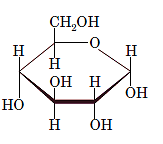
Concept Introduction: A common way of writing structural formula which represent the cyclic structure of monosaccharides is said to be the Haworth projection, which is best represented by chair conformations.
A convention used to represent a 3-D stereo formula in 2-D representation is said to be Fischer projections. In this projection, the vertical lines represent bonds below the plane of the paper and horizontal lines represents bonds above the plane of the paper.
(c)
Answer to Problem 21P
Explanation of Solution
Drawing the Fischer projection of given compound as:
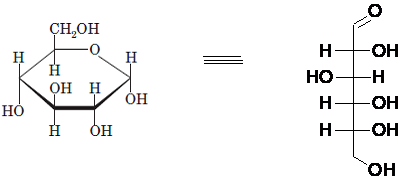
For a D-sugar, the
A new carbon stereocenter created by the conversion of an open structure to a cyclic structure is said to be the anomeric carbon. In carbohydrates, when the hydroxy group on an anomeric carbon is present on the same side as that of the
The anomeric carbon of the given compound is shown by “*”:
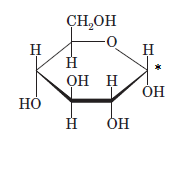
Since, in the given sugar the
Hence, the name of compound is
(d)
Interpretation: The full name along with the anomeric designation of the following compound should be determined:
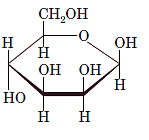
Concept Introduction: A common way of writing structural formula which represent the cyclic structure of monosaccharides is said to be the Haworth projection, which is best represented by chair conformations.
A convention used to represent a 3-D stereo formula in 2-D representation is said to be Fischer projections. In this projection, the vertical lines represent bonds below the plane of the paper and horizontal lines represents bonds above the plane of the paper.
(d)
Answer to Problem 21P
Explanation of Solution
Drawing the Fischer projection of given compound as:
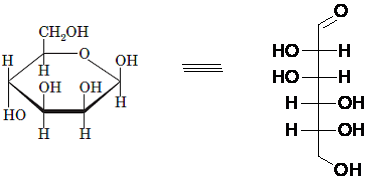
For a D-sugar, the
A new carbon stereocenter created by the conversion of an open structure to a cyclic structure is said to be the anomeric carbon. In carbohydrates, when the hydroxy group on an anomeric carbon is present on the same side as that of the
The anomeric carbon of the given compound is shown by “*”:
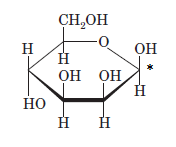
Since, in the given sugar the
Hence, the name of compound is
Want to see more full solutions like this?
Chapter 19 Solutions
Introduction To General, Organic, And Biochemistry
- Which of the following exists in mainly the enol tautomeric form? 요 H3C H3C CH3 i H3C IV || CH 3 EtO H3C ||| OEt OEtarrow_forwardPredict the favored product of each of the following reduction reactions. If the product is chiral, draw both stereoisomers expected. (b) NaBD4 CH3CH₂OH NaBH4 CH3CH₂OHarrow_forward(a) Draw the products (including stereoisomers) formed when 2methylhex-2-ene is treated with HBr in the presence of peroxides. (b) Draw the products (including stereoisomers) formed when (S)-2,4dimethylhex-2-ene is treated with HBr and peroxides under similar conditions.arrow_forward
- Compound A has molecular formula C6H12. Upon treatment with NBS and irradiation with UV light, exactly two constitutional isomers are formed. Which of the following is a possible structure for compound A? OI Oll O III OI and II I and III || |||arrow_forwardDraw the structures and specify the stereochemistry of compounds A-C.arrow_forward(a) What product(s) are formed when the E isomer of C6H5CH = CHC6H5 is treated with Br2, followed by one equivalent of KOH? Label the resulting alkene(s) as E or Z. (b) What product(s) are formed when the Z isomer of C6H5CH = CHC6H5 is subjected to the same reaction sequence? (c) How are the compounds in parts (a) and (b) related to each other?arrow_forward
- yredict the NAME, STRUCTURE and STEREOCHEMISTRY of the BEST ORGANIC REACTANT or the MAJOR ORGANIC product for the following reactions:arrow_forwardA chiral alkyne A with molecular formula C6H10 is reduced with H2 and Lindlar catalyst to B having the R conguration at its stereogenic center. What are the structures of A and B?arrow_forwardA chiral alkyne A with molecular formula C6H10 is reduced with H2 and Lindlar catalyst to B having the R configuration at its stereogenic center. What are the structures of A and B?arrow_forward
- Provide the IUPAC name for the molecule shown here with correct stereochemistry:arrow_forwardRank the compounds in each group in order of increasing reactivity in electrophilic aromatic substitution: (a) C6H6, C6H5Cl, C6H5CHO; (b) C6H5CH3, C6H5NH2, C6H5CH2NH2.arrow_forwardUnder certain reaction conditions, 2,3-dibromobutane reacts with two equivalents of base to give three products, each of which contains two new p bonds. Product A has two sp hybridized carbon atoms, product B has one sp hybridized carbon atom, and product C has none. What are the structures of A, B, and C?arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
