
Organic Chemistry - Standalone book
10th Edition
ISBN: 9780073511214
Author: Francis A Carey Dr., Robert M. Giuliano
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 18.11, Problem 16P
Problem 18.16
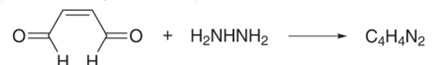
The product of the following reaction is a heterocyclic
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Problem 8.16d
Indicate which of these terms can be used to describe the first step of the following reaction. More than one term may apply.
Bromoetherification, the addition of the elements of Br and OR to adouble bond, is a common method for constructing rings containingoxygen atoms. This reaction has been used in the synthesis of thepolyether antibiotic monensin (Problem 18.34). Draw a stepwisemechanism for the following intramolecular bromoetherification reaction.
Draw the structure of A, an intermediate in the synthesis of theantipsychotic drug risperidone. Explain why three rings in risperidone are considered aromatic.
Chapter 18 Solutions
Organic Chemistry - Standalone book
Ch. 18.1 - Prob. 1PCh. 18.1 - Prob. 2PCh. 18.3 - Prob. 3PCh. 18.4 - Prob. 4PCh. 18.4 - Prob. 5PCh. 18.6 - Prob. 6PCh. 18.7 - Prob. 7PCh. 18.7 - Prob. 8PCh. 18.7 - Prob. 9PCh. 18.8 - Prob. 10P
Ch. 18.8 - Prob. 11PCh. 18.8 - Prob. 12PCh. 18.9 - Prob. 13PCh. 18.10 - Prob. 14PCh. 18.10 - Prob. 15PCh. 18.11 - Problem 18.16 The product of the following...Ch. 18.11 - Prob. 17PCh. 18.12 - Problem 18.18 What other combination of ylide and...Ch. 18.12 - Prob. 19PCh. 18.12 - Prob. 20PCh. 18.12 - Prob. 21PCh. 18.13 - Prob. 22PCh. 18 - (a) Write structural formulas and provide IUPAC...Ch. 18 - Each of the following aldehydes and ketones is...Ch. 18 - The African dwarf crocodile secretes a volatile...Ch. 18 - Prob. 26PCh. 18 - Prob. 27PCh. 18 - Prob. 28PCh. 18 - Prob. 29PCh. 18 - Prob. 30PCh. 18 - Prob. 31PCh. 18 - Each of the following reaction has been reported...Ch. 18 - Prob. 33PCh. 18 - On standing in 17O-labeled water, both...Ch. 18 - Prob. 35PCh. 18 - Prob. 36PCh. 18 - The OH groups at C-4 and C-6 of methyl ...Ch. 18 - Prob. 38PCh. 18 - Prob. 39PCh. 18 - The sex attractant of the female winter moth has...Ch. 18 - Prob. 41PCh. 18 - Prob. 42PCh. 18 - Prob. 43PCh. 18 - Suggest a reasonable mechanism for each of the...Ch. 18 - Prob. 45PCh. 18 - Prob. 46PCh. 18 - Prob. 47PCh. 18 - Prob. 48PCh. 18 - Prob. 49PCh. 18 - Prob. 50PCh. 18 - Prob. 51PCh. 18 - Prob. 52DSPCh. 18 - Prob. 53DSPCh. 18 - Prob. 54DSPCh. 18 - Prob. 55DSPCh. 18 - Prob. 56DSPCh. 18 - Prob. 57DSPCh. 18 - Prob. 58DSP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- HBr 18.2 Give the product.arrow_forwardMuscalure, the sex pheromone of the common housey, can be prepared by a reaction sequence that uses two nucleophilic substitutions. Identify compounds A–D in the following synthesis of muscalure.arrow_forward17.41 Draw the products of each reduction reaction. NABH4 а. CH3OH [1] LIAIH4 [2] H2O HO [1] LIAIHĄ С. [2] H2O b.arrow_forward
- What is the major alkene formed when A is treated with POCl3 andpyridine? Explain why the major product is different in these reactions.arrow_forward18.65 Propose a plausible synthesis for the following trans- formation. CH3 CH3 CH3arrow_forwardMuscalure, the sex pheromone of the common housefly, can be preparedby a reaction sequence that uses two nucleophilic substitutions. Identifycompounds A–D in the following synthesis of muscalure.arrow_forward
- Problem 16.33 What steps are needed to convert benzene to p-isobutylacetophenone, a synthetic intermediate used in the synthesis of the anti-inflammatory agent ibuprofen. ive p-isobutylacetophenone several steps ibuprofen CO₂Harrow_forwardSulfur ylides, like the phosphorus ylides, are usefulintermediates in organic synthesis. Methyl trans-chrysanthemate, anintermediate in the synthesis of the insecticide pyrethrin I,can be prepared from diene A and a sulfur ylide. Draw a stepwisemechanism for this reaction.arrow_forwardMuscalure, the sex pheromone of the common housefly, can be prepared by a reaction sequence that uses two nucleophilic substitutions. Identify compounds A–D in the following synthesis of muscalure.arrow_forward
- Draw the structure of A, an intermediate in the synthesis of the antipsychotic drug risperidone. Explain why three rings in risperidone are considered aromatic.arrow_forward17.27 Predict the product for each of the following reactions: (a) (b) Heat Heat ? ? ? (c) lonete Heat ? loosbyris (d) ? Heat be entarrow_forwardWhen the compound below is treated with a base, kinetic product A and thermodynamic product B,can be formed depending on the base and temperature. Draw the structures for A and B and writethe mechanism for the formation of the thermodynamic productarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
General Chemistry | Acids & Bases; Author: Ninja Nerd;https://www.youtube.com/watch?v=AOr_5tbgfQ0;License: Standard YouTube License, CC-BY