
(a)
Interpretation:
The electron-pair geometry for each carbon atom in
Concept introduction:
The electron pairs in Lewis diagrams repel each other in real molecule and thus they distribute themselves in positions around the central atoms which are far away from one another. This arrangement of electron pairs is called electron-pair geometry. The electron pairs may be shared in covalent bond, or they may be lone pairs.
Answer to Problem 24E
The Lewis diagram for the molecule
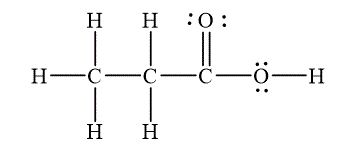
The electron pair geometry for the carbon atoms
Explanation of Solution
To write the Lewis diagram for a compound, first the number of valence electrons is to be calculated. In the molecule,
The atom which is least electronegative and lesser in number is the central atom. In
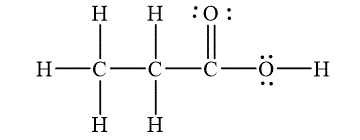
Figure 1
The electron-pair geometry depends on the number of electron pairs around the central atoms. In the molecule
The Lewis diagram for the molecule
(b)
Interpretation:
The molecular geometry predicted by the valence shell electron-pair repulsion theory for each carbon atom in the molecule
Concept introduction:
Molecular geometry is the precise term that is used to describe the shape of molecules and arrangement of atoms around the central atom. The molecular geometry of a molecule is predicted by valence shell electron-pair repulsion theory or in short VSEPR theory. VSEPR theory applies to substances in which a second period element is bonded to two, three, four, or other atoms.
Answer to Problem 24E
The Lewis diagrams for
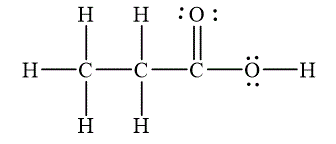
The molecular geometry is tetrahedral for the carbons
Explanation of Solution
To write the Lewis diagram for a compound first the number of valence electrons is to be calculated. In the molecule
The atom which is least electronegative and lesser in number is the central atom. In

Figure 1
The molecular geometry depends on the number of electron pairs as well as number of unpaired electron on the central atoms. In the molecule
The Lewis diagram for the molecule
Want to see more full solutions like this?
Chapter 13 Solutions
Introductory Chemistry: An Active Learning Approach
- QUESTION 13 :C=O: Consider the provided Lewis structure for carbon monoxide: CO. Fill in the blanks with the word "true" or "false" following each of the statements below A non-polar molecule has an even, symmetric distribution of electron density Carbon and oxygen have different electronegativity values. An atom with a lower electronegativity exerts more attraction towards electrons in a shared bond The carbon and oxygen atoms in the CO molecule both attract electrons with the same strength. Carbon monoxide is a polar moleculearrow_forwardQuestion 10 and best indicate degree of polarity. O # of ions and water solubility Mobile phase and stationary phase Dipole moment and dielectric constant Dipole moment and # of ionsarrow_forwardDraw the Lewis Structure for NH2CH2CO2H. Now answer the following questions based on your Lewis structure: (Enter an integer value only.) # single bonds in the entire molecule # double bonds in the entire molecule # lone pairs in the entire moleculearrow_forward
- QUESTION 5 Determine which compound should have a(n) linear molecular geometry. Key Concept: Lewis structures are drawn from a knowledge of the total number of electrons from all the atoms involved in the structure. The element with the lowest electronegativity is the central atom. Fulfill octet of outside atoms first. Molecular shape depends upon the number of atoms and lone pair electrons around the central atom. A H3O+ B ClF2+ C IF2- D AsF5arrow_forwardUse the Molecule Shape simulator (http://openstaxcollege.org/l/16MolecShape) to build amolecule. Starting with the central atom, click on the double bond to add one double bond. Then add one single bond and one lone pair. Rotate the molecule to observe the complete geometry. Name the electron group geometry and molecular structure and predict the bond angle. Then click the check boxes at the bottom and right of the simulator to check your answers.arrow_forwardUse Lewis theory to determine the formula for the compound that forms between each of the following pairs of elements. Ca and Te Express your answer as a chemical formula. Mg and Br Express your answer as a chemical formula. Na and S Express your answer as a chemical formula. In and O Express your answer as a chemical formula.arrow_forward
- Write a correct Lewis structure for carbon monoxide.arrow_forward< Complete the following structural formula for a neutral molecule by adding H atoms to complete the valence of each atom. Do not introduce any double or triple bonds. Then complete the Lewis diagram by adding any unshared electron pairs needed, so that each atom except H has a complete octet. [Review Topics] [References] Use the References to access important values if needed for this question. Br Br C—C— Write the molecular formula in the order CHX, where X stands for Cl or Br. Submit Answer The number of unshared pairs in the Lewis diagram unshared pair(s). Retry Entire Group 9 more group attempts remaining Previous Email Instructor Next Save and Earrow_forwardStep 1 – Write the Lewis structure from the molecular formula.Step 2 – Assign an electron-group arrangement by counting all electron groups (bonding plus nonbonding) around the central atom (or around each centralatom, if more than one central atom in structure).Step 3 – Predict the ideal bond angle from the electron-group arrangement and the effect of any deviation caused by lone pairs or double bonds.Step 4 – Name the molecular shape by counting bonding groups and nonbonding groups separately.Step 5 – Predict whether the molecule is polar or nonpolarStep 6 – Describe the hybridization around the central atom and identify the total number of σ and π bonds in the structurearrow_forward
- Question: For each molecule, draw its shape using Lewis structures and VSEPR theory. Include your steps in creating each sketch. Each diagram should include labelled electronegativities and partial charges for each atom within the molecule, and vector arrows representing the magnitude and direction of each bond dipole and net dipole (for polar molecules). Finally, determine whether the molecule is polar or non-polar and explain your rationale based on bond polarity and molecular shape. CH2Cl2 NI3 CH2O BeF2 SF6 SO2 HBrarrow_forwardTo answer the questions, interpret the following Lewis diagram for NO,Cl. :0-N=0 1. For the central nitrogen atom: The number of lone pairs The number of single bonds The number of double bonds 2. The central nitrogen atom| :O:arrow_forwardMany organic compounds belong to a category of molecules called "hydrocarbons", meaning that they only contain hydrogen and carbons. An example of a simple hydrocarbon is shown below. Considering both the VSEPR shape of the molecule and electronegativity values of the elements and state whether you expect this simple hydrocarbon to be polar or nonpolar. Explain your answer. нн H-C-C-H ннarrow_forward
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning

