
Concept explainers
- 6.
 In the 1950s, a team at Los Alamos National Laboratories built several devices they called “Perhapsatrons,” thinking that PERHAPS they might be able to create controllable nuclear fusion. After several years of experiments, they were never able to maintain a stable plasma and abandoned the project.
In the 1950s, a team at Los Alamos National Laboratories built several devices they called “Perhapsatrons,” thinking that PERHAPS they might be able to create controllable nuclear fusion. After several years of experiments, they were never able to maintain a stable plasma and abandoned the project.
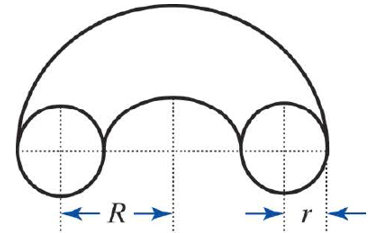
The perhapsatron used a toroidal (doughnut-shaped) plasma confinement chamber, similar to those used in more modern Tokamak fusion devices. You have taken a job at a fusion research lab, and your supervisor asks you to develop a simple spreadsheet to calculate the volume of a torus within which the plasma will be contained in a new experimental reactor.
- a. Create a simple calculator to allow the user to type in the radius of the tube (r) in meters and the radius of the torus (R) in meters and display the volume in cubic meters.
- b. Data validation should be used to assure that R > r in part (a).
- c. Create a table that calculates the volumes of various toruses with specific values for r and R. The tube radii (r) should range from 5 centimeters to 100 centimeters in increments of 5 centimeters. The torus radii (R) should range from 1.5 meters to 3 meters in increments of 0.1 meters.
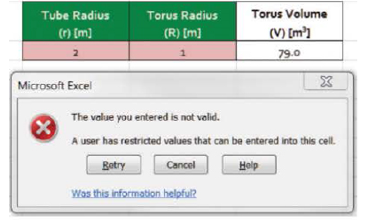 The volume of a torus can be determined using V = 2π2Rr2. A sample worksheet for parts (a) and (b) is shown here.
The volume of a torus can be determined using V = 2π2Rr2. A sample worksheet for parts (a) and (b) is shown here.
Want to see the full answer?
Check out a sample textbook solution
Chapter 10 Solutions
Thinking Like an Engineer: An Active Learning Approach (3rd Edition)
Additional Engineering Textbook Solutions
INTERNATIONAL EDITION---Engineering Mechanics: Statics, 14th edition (SI unit)
HEAT+MASS TRANSFER:FUND.+APPL.
Mechanics of Materials, 7th Edition
DESIGN OF MACHINERY
EBK FUNDAMENTALS OF THERMODYNAMICS, ENH
Engineering Mechanics: Statics & Dynamics (14th Edition)
- a) What is latent heat of vaporization? Latent heat of fusion? b) Assuming a solid material has a specific latent heat of fusion of 334 kJ/kg, what is the energy required to completely melt this material? Mass = 20 kg? c) If the material from part b melts at 200oC and starts at 20oC, what is the total amount of energy required to both bring it to its melting point and melt all of the material? (Cp = 18 kJ/kgK)arrow_forwardMaterial A with initial temperature of 80 ⁰C came into contact with material B with initial temperature of 15 ⁰C. What are the scenario/s that would likely to happen?Temperature of material B will be above 80 ⁰CEntropy of material A will increaseEnergy transfer is from material B to material ATemperature of material A will be below 15 ⁰CA.) If all 4 statements are trueB.) If 3 of the 4 statements are trueC.) If 2 of the 4 statements are trueD.) If only 1 of the 4 statements is trueE.) If none of the 4 statements is truearrow_forward26. Please help me answer all parts to this physics questionarrow_forward
- a) What is the continuum assumption, and when does the continuum assumption break down? b) Define the Knudsen number, and what is its physical significance? c) What are the unique issues related to microfluidics? d) What are the applications of microfluidics?arrow_forwardA:What happens for 01,02 ,v2 ,v when a beam of light travels from air to water ?Why? B: You are approached by a man on the street who offers to sell you a rather impressive ?genuine diamond .?He produces a certificate of authenticity while telling you of the hardships forcing him to part with his wife's ? engagement stone (a family heirloom). Being skeptical, you decide to verify the composition of the stone with the laser pointer attached to your key ring. (a) What is the critical angle for a diamond/air interface? For a glass/air interface? (b) How could you use this information to determine the composition of the stone?Were that n(diamond)=2.4 ,n(glass)=1.5,n(air)=1arrow_forwardAccording to the Bohr model of the atom a. electrons in orbit around nuclei lose energy very slowly b. electrons around a nucleus can have only certain particular energies and can only occupy certain specific orbits at particular distances from the nucleus c. quantum theory is not applicable to the ultra-structure of an atom d. all of the options are truearrow_forward
- STEP 2 Your first customer asked for an air piston-cylinder to use it in air compressors for generating high pressure. Your role in your company is to analyze, describe the operation of the system and their properties, explain and demonstrate the application of the first law of themodynamics to this system, as well as, illustrate the importance of expressions for work done in the system through the application of the first law. Describe the operation of the themodynamic system in Figure 1 and its properties, by constructing a P-V and T-V plots showing the process, the path, and the states then show the work under the curve on the P-V plot. STEP 3 Explain the application of the first law of thermodynamics to the system and explain the system parameters in terms of energy forms changes using the non-flow energy equation (NFEE) for the system and how they affect other parameters such as the final air temperature, by stating the appropriate assumptions and writing the general formula and…arrow_forwardQuantum mechanicsarrow_forwardQuestion 6 Which of the following is an example of thermal technology? An engine Cell phones The furnace in your hosue Lasersarrow_forward
- The objectives of this experimentarrow_forwardImagine an alternate universe where the value of the Planck constant is 6.62607 x 10 40 J-s. In that universe, which of the following objects would require quantum mechanics to describe, that is, would show both particle and wave properties? Which objects would act like everyday objects, and be adequately described by classical mechanics? object quantum or classical? classical A human with a mass of 65, ko. 2.2 m high, moving at 3.0 m/s. O quantum O dassical A ball with a mass of 195. 0. 8.7 cm wide, moving at 35.1 m/s O quantum O dlassical A raindrop with a mass of 33.0 mg. 4.5 mm wide. moving at 6.4 m/s. O quantum O dassical An alpha partide with a mass of 6.6x 102 kg, 8.0 x 10 15 m wide, moving at 17. kmis: OQuantumarrow_forward(c) A hardboard wall is exposed to a window on a neighbouring property which is 2 metres high x 2 metres wide and located 2 metres from the hardboard wall. The temperature inside the room behind the window is 900 °C and a glowing ember from the fire lands on the hardboard wall to act as a pilot. Will the hardboard wall ignite? Show your working.arrow_forward
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY





