
Concept explainers
Consider the Maxwell-Boltzmann distribution function plotted in Problem 28. For those parameters, determine the rms velocity and the most probable speed, as well as the values of f(v) for each of these values. Compare these values with the graph in Problem 28.
28. Plot the Maxwell-Boltzmann distribution function for a gas composed of nitrogen molecules (N2) at a temperature of 295 K. Identify the points on the curve that have a value of half the maximum value. Estimate these speeds, which represent the range of speeds most of the molecules are likely to have. The mass of a nitrogen molecule is 4.68 × 10−26 kg.
Equation 20.18 can be used to find the rms velocity given the temperature, Boltzmann’s constant, and the mass of the atom or molecule. The mass of a nitrogen molecule is 4.68 × 10−26 kg.
Using the results of Problem 28 and the rms velocity, we can calculate the value of f(v).
f(vrms) = (3.11 × 10−8)(511)2 e
The most probable speed, for which this function has its maximum value, is given by Equation 20.20.
f(vmp) = (3.11×10−8)(417)2 e−
We plot these points on the speed distribution. The most probable speed is indeed at the peak of the distribution function. Since the function is not symmetric, the rms velocity is somewhat higher than the most probable speed.
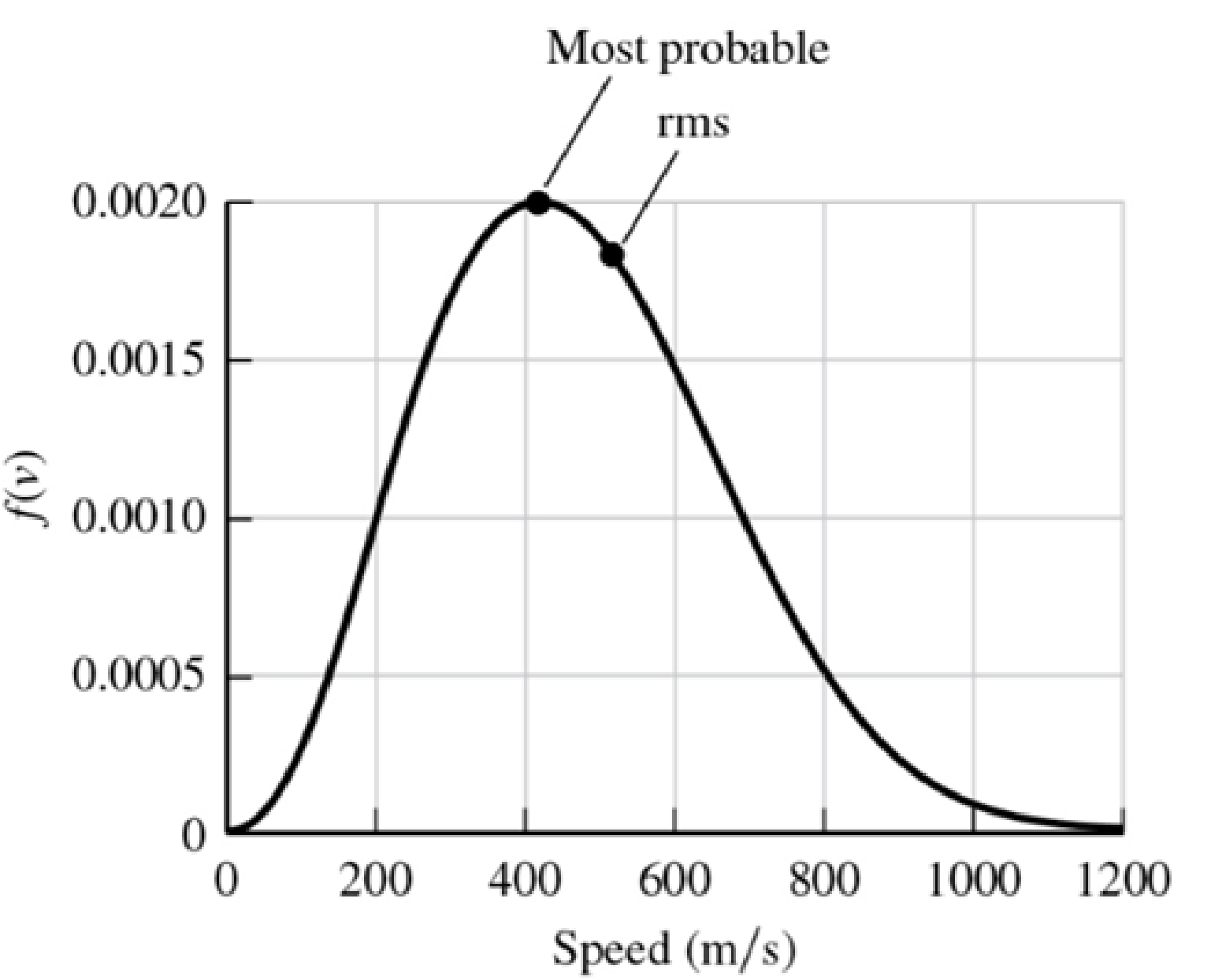
Figure P20.29ANS
Want to see the full answer?
Check out a sample textbook solution
Chapter 20 Solutions
Physics for Scientists and Engineers: Foundations and Connections
- In the text, it was shown that N/V=2.681025m3 for gas at STP. (a) Show that this quantity is equivalent to N/V=2.681019cm3, as stated. (b) About how many atoms are mere in one m3 (a cubic micrometer) at STP? (c) What does your answer to part (b) imply about the separation of Mama and molecules?arrow_forwardProblem 6: There are lots of examples of ideal gases in the universe, and they exist in many different conditions. In this problem we will examine what the temperature of these various phenomena are. Part (a) Give an expression for the temperature of an ideal gas in terms of pressure P, particle density per unit volume ρ, and fundamental constants. T = P/( ρ kB ) Part (b) Near the surface of Venus, its atmosphere has a pressure fv= 96 times the pressure of Earth's atmosphere, and a particle density of around ρv = 0.92 × 1027 m-3. What is the temperature of Venus' atmosphere (in C) near the surface? Part (c) The Orion nebula is one of the brightest diffuse nebulae in the sky (look for it in the winter, just below the three bright stars in Orion's belt). It is a very complicated mess of gas, dust, young star systems, and brown dwarfs, but let's estimate its temperature if we assume it is a uniform ideal gas. Assume it is a sphere of radius r = 5.8 × 1015 m (around 6 light years)…arrow_forwardThe equipartition theorem states that each term in the particle's energy depending on a squared position (potential energies) or velocity (kinetic energies) contributes on average kT to the particle's total mechanical energy. Each of these terms corresponds to a degree of freedom of the gas. That is, 6. By what factor do you have to increase the temperature to triple the rms speed of an ideal gas?arrow_forward
- An expensive vacuum system can achieve a pressure as low as 1.00 x 10-7 N/m² at 25.5 °C. How many atoms N are there in a cubic centimeter at this pressure and temperature? The Boltzmann constant k = 1.38 x 10-23 J/K. N = atomsarrow_forwardI just need help with part D Problem 6: There are lots of examples of ideal gases in the universe, and they exist in many different conditions. In this problem we will examine what the temperature of these various phenomena are. Part (a) Give an expression for the temperature of an ideal gas in terms of pressure P, particle density per unit volume ρ, and fundamental constants. Answer: T = P/( ρ kB ) Part (b) Near the surface of Venus, its atmosphere has a pressure fv= 96 times the pressure of Earth's atmosphere, and a particle density of around ρv = 0.92 × 1027 m-3. What is the temperature of Venus' atmosphere (in C) near the surface? Answer: Tv = 490.55 Part (c) The Orion nebula is one of the brightest diffuse nebulae in the sky (look for it in the winter, just below the three bright stars in Orion's belt). It is a very complicated mess of gas, dust, young star systems, and brown dwarfs, but let's estimate its temperature if we assume it is a uniform ideal gas. Assume it is a…arrow_forwardA bottle of volume V = 0.15 m³ contains helium gas (a monatomic gas) at a pressure p = 722,266 Pa (Pascal = N/m² and temperature T = 300 K. Calculate a numerical value for the internal energy U of this gas. Include units in your answer, using Sl units (m for meters, kg for kilograms, s for seconds, J for joules, K for kelvin, etc.). Write your answer as an exponential as described in the instructions.arrow_forward
- You are studying a gas known as "gopherine" and looking in the literature you find that someone has reported the partition function for one molecule of this gas, 5/2 AzT q(V, T) = ) %3D h?m Assume that the molecules are independent and indistinguishable. Derive the expressions for the energy, (E), for this gas. Give your answers in terms of N, kg, T. V and the constants A and B. O (E) = NkaT ㅇ (E) =D NkaT ㅇ (E) %3D NkaT- O (E) = ANKET - O (E) = - T ㅇ (E)=D 쑤-arrow_forwardA)An ideal gas is confined to a container at a temperature of 330 K.What is the average kinetic energy of an atom of the gas? (Express your answer to two significant figures.) B)2.00 mol of the helium is confined to a 2.00-L container at a pressure of 11.0 atm. The atomic mass of helium is 4.00 u, and the conversion between u and kg is 1 u = 1.661 ××10−27 kg.Calculate vrmsvrms. (Express your answer to three significant figures.) C)A gold (coefficient of linear expansion α=14×10−6K−1α=14×10−6K−1 ) pin is exactly 4.00 cm long when its temperature is 180∘∘C. Find the decrease in long of the pin when it cools to 28.0∘∘C? (Express your answer to two significant figures.)arrow_forwardA cylinder of diameter S, of height h, contains pure gas with equation PV = nRT at constant temperature T_0. The z axis is directed upwards and the gravitational field is assumed to be uniform. 1) Using the fundamental principle of hydrostatic statistics, show that dp = -pgdz where p = p (z) is the gas pressure at height z. 2) If P_0 is the gas pressure at the foot of the pole, calculate the pressure p (z) at height z. 3) In the case of wind (M = 29 g / mol: R = 8.31J/ mol.k) at temperature T_0 = 300K, calculate the height of the poles necessary to observe the change in pressure (pressure at the threshold) at 5% .arrow_forward
- Suppose you have an ideal gas so that pV=NkT. Use the Maxwell Relations to find a solution to the partial derivative: -(∂S/∂p)T,N. Match the partial derivative to the correct solution below. a. kT/p b. p c. p/nK d. kT e. Nk/p f. p/kT g. NkTarrow_forwardProblem 1: a) Calculate the most probable speed, average speed, and rms speed for nitrogen N, at room temperature. b) Use a computer to plot the Maxwell speed distribution for nitrogen molecules at T=300K and T=600K. Plot both as line graphs on the same axes and label the axes. c) At room temperature, what fraction of the nitrogen molecules in the air are moving at less than 300m/s?arrow_forwardConsider a gas composed of 1 mol of deuterium atoms (molar mass = 2 g/mol) in a 0.025 m³ container at a temperature of 400 K. Using the results of statistical mechanics, calculate the Helmholtz energy of the gas in J.arrow_forward
 Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College
College PhysicsPhysicsISBN:9781938168000Author:Paul Peter Urone, Roger HinrichsPublisher:OpenStax College

