
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN: 9780618974122
Author: Andrei Straumanis
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter L4, Problem 5E
For each structure below, use numbers to indicate chemically equivalent and distinct hydrogens,and make a table showing the predicted integration and multiplicity of each peak cluster.
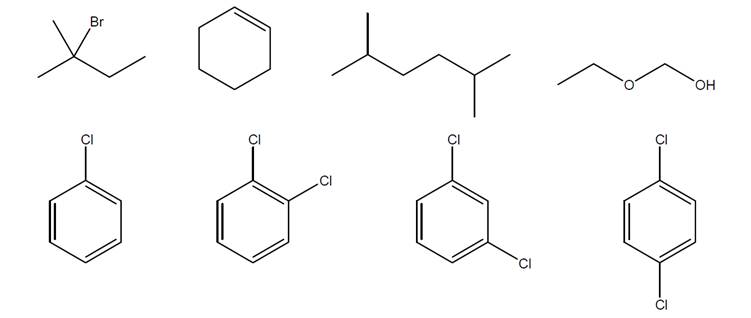
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
3. Look at the two spectra below, one of which is 2-methylcyclohexanol and one of which is
3methylcyclohexene. Which spectrum belongs to which compound? Explain your reasoning.
10-
4000
200
3500
3000
2500
1500
1000
600
Spectrum A
90
20
30
20
10
500
3000
2500
2000
1600
1000
500
Vieters kn1
Spectrum B
Given is the mass spectra of n-octane whre peaks are labeled accordingly. Give the letter that corresponds to the peak described below:
CH3-CH2-CH2-CH2-CH2-CH2-CH2-CH3
Which peak is considered as the base peak?
Which is peak is the Molecular ion peak?
Which the isotopic peak of the molecular ion?
Which peak reperesents the (CH3-CH2-CH2-CH2)+ fragment?
Which is the methyl ion peak?
5.
For each structure below, use numbers to indicate chemically equivalent and distinct hydrogens
and make a table showing the predicted integration and multiplicity of each peak cluster.
Br
Y
OH
""OH
CI
CI
d
OH
OH
xox
Chapter L4 Solutions
Organic Chemistry: A Guided Inquiry
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Following are infrared spectra of nonane and 1-hexanol. Assign each compound its correct spectrum.arrow_forwardFollowing are mass spectra for the constitutional isomers 2-pentanol and 2-methyl- 2-butanol. Assign each isomer its correct spectrum.arrow_forward1) Indicate chemically equivalent nuclei (e.g. Ha, H³, Hc). 2) Estimate the chemical shifts on the following hydrogens. 3) Indicate the ratio of the area under the peaks 4) Indicate the Spin Spin splitting (singlet, doublet, triplet, etc) 1, 1, 2 tribromoethane Ethyl benzene Methyl isopropyl ketone Isopropyl propionatearrow_forward
- Why is this the correct spectra for these molecules. Identify a specific absorption band which identifies each characteristic functional group of the molecule chosen.arrow_forward1. Identify the important peaks, give an analysis about what the compound may look like 2. Identify electronic transitions (in \( \mathrm{MeOH} \) )arrow_forwardCalculate the IHD then identify the important peaks in the following MS spectral data and draw the structure of the important peaks in the following MS spectral data. Typewritten please because I have a poor eyesight that's why I'm having a hard time understanding handwritten answer especially cursive. Thank you for understanding.arrow_forward
- How many 13C peaks should be seen in the broad-band decoupled spectrum of the structure at the right?arrow_forwardI am having trouble with the park where we determine what kind of peak each group of H's are. I am confused about counting nieghbors. Do I count every carbon thats right next to each carbon , how far do I count? Like for the group I that I marked with purple, I counted 3 adjacent carbons, but it says its a triplet. And the one labeled as a nonet ( 9 peaks ) , I dont understand where 9 came from. Can someone explain how to correctly count neighbors?arrow_forwardIn the attached H1-NMR, draw and identify each set of equivalent hydrogens in the structure and list peak they belong to in the NMR spectrum.arrow_forward
- Why are the spectra of 1,4-cyclohexanedione and 1,2-cyclohexanedione different?arrow_forwardCH3 CH3 O of OH H3C- protons it represents. letter -OCH₂CH3 For each of the above compounds, do the following: 1. List the wave numbers of all the IR bands in the 1350-4000 cm-1 region. For each one, state what bond or group it represents. d value H₂C CH3 H₂C H splitting 2. Label equivalent sets of protons with lower-case letters. Then, for each ¹H NMR signal, give the 8 value, the type of splitting (singlet, doublet etc.), and the number H # of protons O 3. Redraw the compound and label equivalent sets of carbons with lower-case letters. -OCH3 Then for each set of carbons give the & value and # of carbons it represents. letter d value # of carbonsarrow_forwardPlease discuss this in a paragraph. Which mass spectrum belongs to which compound? - differences in fragmentation in isomers- Assign each spectrum to one compound Justify your assignment by assigning relevant signals in each spectrum. Explain how you could determine which spectrum belongs to which compound.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning,
Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning,

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Physical Chemistry
Chemistry
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Wadsworth Cengage Learning,
IR Spectroscopy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=_TmevMf-Zgs;License: Standard YouTube License, CC-BY