
Concept explainers
Interpretation:
Reagents used are to be suggested in each part.
Concept introduction:
The reaction which occurs in between two esters or one ester and another carbonyl compound, and forms a carbon–carbon bond, in the presence of a strong base, leading to the formation of a β-keto ester or a β-diketone is known as Claisen condensation.
The removal of carbon dioxide from a carbonyl compound is termed as a decarboxylation reaction.
Sodium borohydride
Hydrogen bromide
Answer to Problem 1PP
Solution:
(a)
Sodium ethoxide
(b)
An acid
(c)
Heat
(d)
Sodium borohydride
(e)
Hydrogen bromide
(f)
Base
Explanation of Solution
The first step is similar to a crossed Claisen condensation. The reagent used in this reaction is sodium ethoxide. The molecular formula of sodium ethoxide is
The reaction shown is as follows:
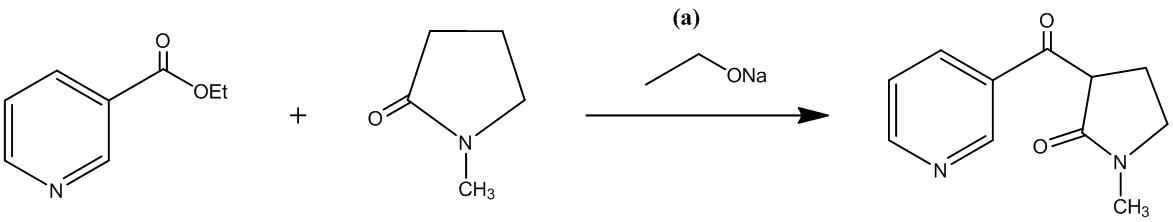
The second step involves the hydrolysis of an amide that is lactamide, which can be carried out with either an acid or a base. In the given reaction the desired product is formed by using an acid.
The reaction is shown as follows:
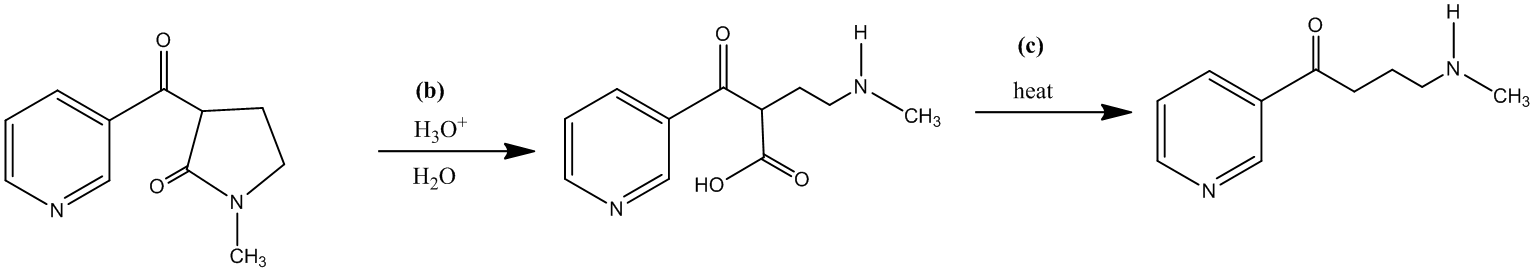
The third step is the decarboxylation of a β-keto acid, which is formed by the acidic hydrolysis of an amide. It requires only the application of heat to get the desired product. The reaction takes place along with the second step.
The forth step is the reduction of the ketone, obtained in third step, into a secondary alcohol. There are many reducing agents, which can reduce a ketone into an alcohol, for instance, sodium borohydride. The molecular formula for the same is
The reaction shown is as follows:
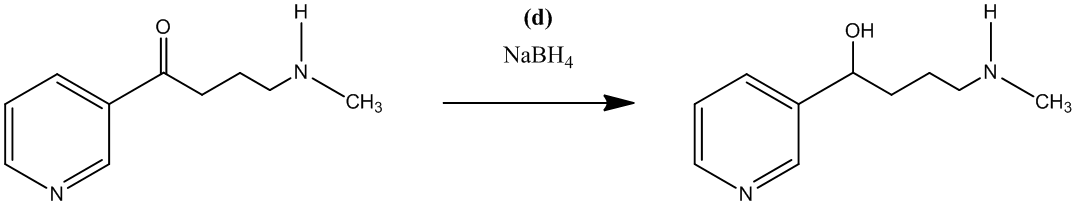
The fifth step is the conversion of the secondary alcohol, obtained in forth step, to an alkyl bromide by using hydrogen bromide. This reagent also gives a hydrobromide salt of the aliphatic
The reaction shown is as follows:
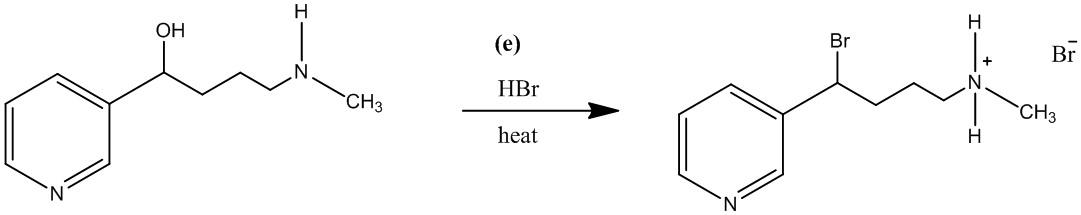
The treatment of the salt with base will lead to the formation of the secondary amine. It will further act as a nucleophile and attack the carbon atom bearing the bromine. This reaction leads to the formation of a five-membered ring and (±) nicotine.
The reaction is shown as follows:
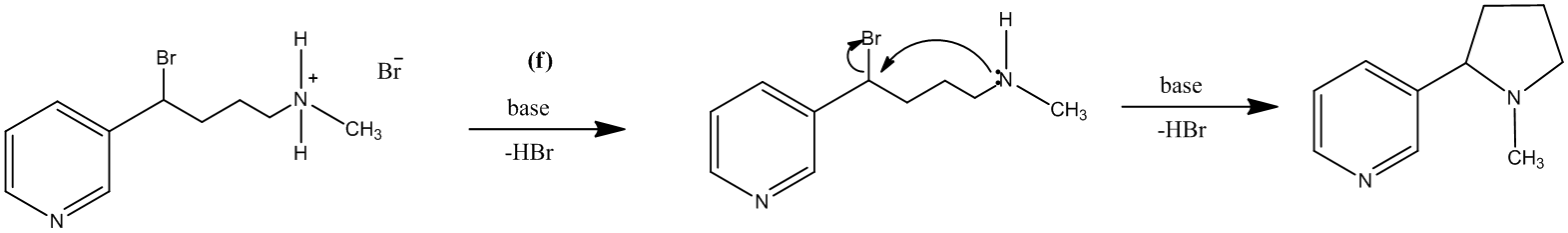
The reagents that could be used for each part are suggested as:
(a): Sodium ethoxide
(b): An acid
(c): Heat
(d): Sodium borohydride
(e): Hydrogen bromide
(f): Base
Want to see more full solutions like this?
Chapter H Solutions
Organic Chemistry
- 9) Which of the following pairs will form only one product of aldol addition, if the second component will be slowly added to the reaction mixture? Answer A) which CH3 H3C H3C. H3C B) H3C. H3C CH3 H H3C. D) H3C. CH3 H3C H CH3 + H3C CH3 thaich CH3 H3C CH3 l H CH3 Harrow_forwardPropose a synthesis of the product below starting from the provided starting materials. All the carbons in the product must come from the given starting materials. The starting materials can be used more than once. You may use any other necessary reagent. Show each step and the product that would be formed. I need help with this practice problem, thank you!arrow_forwardProblem 3 of 14 Draw the major product of this reaction. Ignore inorganic byproducts. HBr (excess) Submit Select to Drawarrow_forward
- 7:07 Problem 5 of 15 Draw the major product of this reaction. Ignore inorganic byproducts. 1. LIAIH4 2. H3O* NO₂ Select to Draw Submit €arrow_forwardProblem 18 of 50 Submit Curved arrows are used to illustrate the flow of electrons. Follow the arrows and draw the product formed in this reaction. Include all lone-pairs. Ignore any inorganic byproducts. HNO3 H2SO4 مل علم Нarrow_forwardProblem 2,8 Suggest a plausible arrow-pushing mechanism for the following tautomerization reactions cat. HA OH cat B O NH2 NH2 NIHarrow_forward
- Problem Three a) Identify the following as nucleophile or electrophile: iii) C,H,CHO vi) HOC₂H₂ i) C,H5NH, iv) (C$Hs)₂CO ii) (C₂Hs)3N v) (C₂H5)₂CH+ viii) CH,SH vii) CN ix) CH₂CH₂CH₂CH₂ Br b) i) Draw the curly arrow mechanism for the reaction between v) and vii) x) (CH3)3CBr ii) of the two C atoms involved do any change their hybridisation? If so, how?arrow_forwardProblem 1. Propose a reasonable synthesis for the following compound from methylcyclohexane. (Hint: can be done in three steps) CH3 ??? CH3 desired product D = deuterium (²H)arrow_forwardPredict the major product of the following reactions. The formulae of the products are given in bold. Please answer d,e,f,garrow_forward
- Problem 5 of 20 Draw the product of the E2 reaction shown below. Ignore any inorganic byproducts. Br KOtBu Draw the E2 Product Tap here for additional resources Submit Oarrow_forward(please correct answer and don't use hend raiting) Predict the product of the following reactions and show the reaction mechanismarrow_forward6) Predict the product. H H EtQ: Na +7) Show curved arrow mechanism of the formation of the product. EtQ : + Na 8) Is the above reaction favorable or not? marrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
