
EBK ORGANIC CHEMISTRY-PRINT COMPANION (
4th Edition
ISBN: 9781119776741
Author: Klein
Publisher: WILEY CONS
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 9.3, Problem 2LTS
Interpretation Introduction
Interpretation:
It is necessary to determine whether sodium acetate
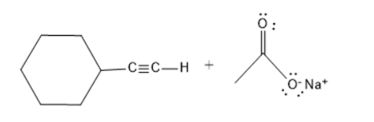
Concept Introduction :
The amount of hydrogen ions that one acid molecule can produce at most is one indicator of an acid's basicity. Due to its capacity to shed one proton or hydrogen atom, acetic acid is monobasic in nature.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Draw a resonance structure, complete with all formal charges and lone (unshared) electron pairs, that shows the resonance
interaction of the acetoxy with the para position in phenyl acetate.
phenyl acetate
●
CH3
• You do not have to consider stereochemistry.
• Include all valence lone pairs in your answer.
In cases where there is more than one answer, just draw one.
Sn [F
?
12) Use the curved arrow formalism to show the movement of electron pairs in the
following reaction and label each reactant as a nucleophile or an electrophile.
CHÍNH CHỊCH, + CO
Học Nha
CH₂CH₂CI
Draw a structural formula(s) for the major organic product(s) of the following reaction.
Br + CH3CH₂O™ Na*
ethanol
• Use the wedge/hash bond tools to indicate stereochemistry where it exists.
• You do not have to explicitly draw H atoms.
• If a group is achiral, do not use wedged or hashed bonds on it.
• Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right corner.
Separate multiple products using the + sign from the drop-down menu.
Chapter 9 Solutions
EBK ORGANIC CHEMISTRY-PRINT COMPANION (
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Which of the following aldehydes has the highest solubility in water? A) CH₃CH₂C(=O)H B) CH₃CH₂CH₂CH₂C(=O)H C) CH₃(CH₂)₅C(=O)H D) CH₃(CH₂)₁₀C(=O)H E) All of these have equal solubility.arrow_forwardCH=CHNO₂ (2-nitrovinyl)benzene Electrophilic substitution on (2-nitrovinyl)benzene occurs at the meta position. Draw resonance structures to show how the ring is electron-poor at the ortho and para positions. You do not have to consider stereochemistry. • Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right corner. • Separate resonance structures using the symbol from the drop-down menu. 0 78 00-F karrow_forwardSuppose the alkene in the drawing area below is put in strong acid solution, for example a solution of HBr. Highlight each carbon that might become protonated to form a carbocation intermediate. (The carbocation intermediate might then react with something else to form a final product.) سعدarrow_forward
- Markonikov's rule states that with the addition of a protic acid HX to an asymmetric alkene, the H or electropositive part gets attached to the carbon with more alkyl substituents, and the X group or electronegative part gets attached to the carbon with more hydrogens. O True O Falsearrow_forwardUse the dropdown menus to choose the correct name for each structure shown. V [ Select ] H. 2-methylbenzoic acid CH3 ethylcyclocarboxylic acid 2-methylcyclocarboxylic acid 2-ethylbenzoic acid ( Select ) -C-OH O CH, -CH,-C [ Select] CH, | Select ) CH,-CH-CH2-c-o-CH, CH-CH-NH-CH, CH, | Select ) H3C CH3 'N | elect ] Use the dropdown menus to choose the correct name for each structure shown, ( Select ) H. CH3 COH V[ Select ) 2,2,2-trichloroethanoicacid 1,1,1 trichloro-2-carboxyethane 1, 2,2 chloroethanoic acid 2,2,2-chloroethanoic acid O CH, но---сн,-сн,--он [Select CH, ( Select ] CH-CH, -CH2-č-o-CH, CH,-CH-NH-CH, [ Select ] CH, H3C CH3 [ Select [ Select) H. CH3 [ Select) -c-OH CH, HO-E-C-CH,-CH, CH, OH [Select ) 2,2 dimethyl-1,5-pentanedioic acid 3,3-dimethylpentanedioic acid 2,2-dimethylpetanoic acid CH,-CH, -CH2-c-o-CH 2,2-dimethylpetanedioic acid CH,-CH-NH-CH, CH, ( Select] H3C CH3 'N' [ Select )arrow_forward4. Write in the product of this reaction: H₂C CH₂ LIAIHarrow_forward
- Draw the product(s) of the following reactions. CH3(CH₂)7-CEC-(CH₂)7-C Y [*** OH • Consider E/Z stereochemistry of alkenes. • If no reaction occurs, draw the organic starting material. • Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right corner. Separate multiple products using the + sign from the drop-down menu. H₂C=CH₂ H₂ Lindlar catalyst O $ [ ] كر [References] ChemDoodle Prevarrow_forwardA certain hydrocarbon had the molecular formula C16H26 and contained two triple bonds. Ozonolysis gave CH3(CH2), CO₂H and HO₂CCH2CH2CH2CH2CO₂H as the only products. Draw a reasonable structure for this hydrocarbon. Click and drag to start drawing a structure. D:arrow_forward4:32 Done 13. Hydrogenation of glyceryl trioleste, a triacylglycerol made from three oleic acids and glycerol, Converts the ester group to a ketone group. 14. The products of the acid catalyzed hydrolysis of a fat are A) the esters of fatty acids. the ester group to a carboxylic acid group. the alkene group into an alkane group. converts the ester group into the alkene group. B) fatty acids and glycerol. C) salts of fatty acids. D) salts of fatty acids and glycerol. E) phospholipids. 15. In the list below, which lipid type is most soluble in water? A) triacylglycerols B) glycerophospholipids C) oils D) steroids E) waxes (+) CHEM 6 o 16. The type of lipid that gives a cell membrane its shape is a A) triacylglycerol. B) glycerophospholipid. C) prostaglandin. D) bile salt. E) WEL 17) Which of the following contains a-1,6- branches? A) amylose B) glycogen C) cellulose D) sucrose E) maltose A LANG 4 18. Lipids are compounds that are soluble in A) distilled water. B) normal saline solution.…arrow_forward
- Draw a structural formula of an alkene or alkenes (if more than one) that undergo acid-catalyzed hydration and without rearrangement give 2-methyl-2-butanol as the MAJOR product. You do not have to consider stereochemistry. You do not have to explicitly draw H atoms. If more than one structure fits the description, draw them all. Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right corner. Separate structures with + signs from the drop-down menu.arrow_forwardDraw the product(s) of the following reactions. H H3C-CEC-CH₂CH₂-C H C-CH3 H₂ Pd/C • Consider E/Z stereochemistry of alkenes. • If no reaction occurs, draw the organic starting material. • Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right corner. • Separate multiple products using the + sign from the drop-down menu.arrow_forward2. Draw structures for the following systems. If more than one isomer is possible, draw strcutures for all the possible isomers. Benzene ring with a methyl group and a nitro group (Draw all possible isomers, and name them) Benzene ring connected to a heptane chain (Draw all possible isomers, and name them) 2-benzyl-3- methylbutan-1-olarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY