
A gas turbine operates with a regenerator and two stages of reheating and intercooling. Air enters this engine at 14 psia and 60°F, the pressure ratio for each stage of compression is 3, the air temperature when entering a turbine is 940°F, the engine produces 1000 hp, and the regenerator operates perfectly. The isentropic efficiency of each compressor is 88 percent and that of each turbine is 93 percent. Which process of the cycle loses the greatest amount of work potential? The temperature of the heat source is the same as the maximum cycle temperature, and the temperature of the heat sink is the same as the minimum cycle temperature. Use constant specific heats at room temperature.
Which process of the cycle loses the greatest amount of work potential.
Answer to Problem 151P
The exergy destruction associated with process 1-2 and 3-4 is
The exergy destruction associated with process 5-6 and 7-8 is
The exergy destruction associated with process 6-7 and 8-9 is
The exergy destruction associated with process 10-1 and 2-3 is
The exergy destruction associated at regenerator is
During the heat rejection process the highest energy destruction occurs.
Explanation of Solution
Draw the
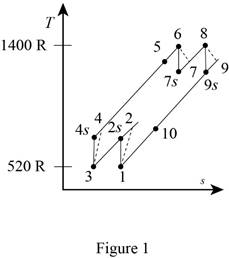
Write the expression for the temperature and pressure relation for the isentropic process 1-2s.
Here, the pressure ratio is
Write the expression for the efficiency of the compressor
Here, the specific heat at constant pressure is
Write the expression for the temperature and pressure relation ratio for the expansion process 6-7s.
Here, temperature at state 7s for isentropic process is
Write the expression for the efficiency of the turbine
Here, temperature at state 7 is
Write the expression to calculate the heat input for the two-stage gas turbine
Here, the specific heat of air at constant pressure is
Write the expression to calculate the heat output for the two-stage gas turbine
Write the expression for the exergy destruction during the process of as steam from an inlet to exit state.
Here, entropy generation is
Write the expression of exergy destruction for process 1-2
Here, pressure at state 2 is
Write the expression of exergy destruction for process 5-6
Here, pressure at state 5 is
Write the expression of exergy destruction for process 6-7
Here, pressure at state 7 is
Write the expression of exergy destruction for process 10-1
Here, pressure at state 10 is
Write the expression of exergy destruction for regenerator
Conclusion:
Substitute
Substitute
Substitute
Substitute
The regenerator is ideal, the effectiveness is 100% and therefore,
Substitute
Substitute
Substitute
Thus, the exergy destruction associated with process 1-2 and 3-4 is
Substitute
Thus, the exergy destruction associated with process 5-6 and 7-8 is
Substitute
Thus, the exergy destruction associated with process 6-7 and 8-9 is
Substitute
Thus, the exergy destruction associated with process 10-1 and 2-3 is
Substitute
Thus, the exergy destruction associated at regenerator is
During the heat rejection process the highest energy destruction occurs.
Want to see more full solutions like this?
Chapter 9 Solutions
Thermodynamics: An Engineering Approach
- The adiabatic efficiencies of the compressor and turbine used in an air-standard Brayton cycle are 85% and 90%, respectively. If the cycle operates between 14.7 and 55 psia and if the maximum and minimum temperatures are 1500 F and 80 F, respectively, compute the thermal efficiency of the cycle. Assume constant specific heats.arrow_forwardRequired information Consider an ideal gas-turbine cycle with two stages of compression and two stages of expansion. The pressure ratio across each stage of the compressor and turbine is 3. The air enters each stage of the compressor at 300 K and each stage of the turbine at 12o0 K. Use variable specific heats. Determine the back work ratio and the thermal efficiency of the cycle, assuming a regenerator with 80 percent effectiveness is used. The back work ratio is %. The thermal efficiency is %.arrow_forwardIn an air standard dual cycle two –thirds of the total heat supply occurs at constant volume. The state at the beginning of the compression process is 90kPa and 20°C and the compression ratio is 9. if the total heat supply is 2100kJ/kg, Determine the maximum pressure of the cycle and maximum temperature of the cycle.arrow_forward
- The compression ratio of an air-standard Otto cycle is 9.5. Assume the start of isentropic (reversible adiabatic) compression is state point 1. Prior to the isentropic compression process, the air is at 100 kPa, 35°C, and 600 cm3. The temperature at the end of the isentropic (reversible) expansion process is 800 K. Take the average values of the specific heat capacities, cP = 1.005 kJ/(kg·K) and cv = 0.718 kJ/(kg·K). (a) Draw the Otto cycle on both the P-v and T-s diagrams. Determine the: (b) highest temperature (in K) and pressure (in kPa) in thecycle, (c) amount of total heat transferred in, inkJ, (d) thermal efficiency, and (e) mean effective pressure in kPa.arrow_forwardin an air standard dual cycle two –thirds of the total heat supply occurs at constant volume . The state at the beginning of the compression process is 90kPa and 20°C and the compression ratio is 9. if the total heat supply is2100kJ/kg, (a) determine the maximum pressure of the cycle (b) determine the maximum temperature of the cyclearrow_forwardThe compression ratio of an air-standard Otto cycle is 9.5. Assume the start of isentropic (reversible adiabatic) compression is state point 1. Prior to the isentropic compression process, the air is at 100 kPa, 35°C, and 600 cm3. The temperature at the end of the isentropic (reversible) expansion process is 800 K. Take the average values of the specific heat capacities, cP = 1.005 kJ/(kg·K) and cv = 0.718 kJ/(kg·K). (a) Draw the Otto cycle on both the P-v and T-s diagrams. Determine the: (b) highest temperature (in K) and pressure (in kPa) in the cycle, (c) amount of total heat transferred in, in kJ, (d) thermal efficiency, and (e) mean effective pressure in kPa.arrow_forward
- A turboprop aircraft propulsion engine operates where the air is at 8 psia and −10°F, on an aircraft flying at a speed of 600 ft/s. The Brayton cycle pressure ratio is 10, and the air temperature at the turbine inlet is 940°F. The propeller diameter is 10 ft and the mass flow rate through the propeller is 20 times that through the compressor. Determine the thrust force generated by this propulsion system. Assume ideal operation for all components and constant specific heats at room temperature.arrow_forwardDiesel engine takes in atmospheric air at 101.325 kPa and 29°C. The initial volume of the air is 0.6 m³. The compression ratio is 15 and the cut-off occurs at 6% of the stroke. The engine completes a cycle in 2 revolutions, and is running at an engine speed of 3000 rpm. Determine the following: a. Power, in kW b. Efficiencyarrow_forward2) A heat addition is 1500 kJ/kg of air for an ideal Otto cycle which has a compression ratio of 8. Moreover, pressure and temperature at the beginning of the compression process are 85 kPa and 10°C. Assuming constant specific heat ratio as 1.4. Determine the maximum pressure and temperature (10) of the cycle, the thermal efficiency (12,5) of the cycle and the mean effective pressure (12,5). (cv=0.717, R=0.287) (35p)arrow_forward
- 2. Consider an ideal gas-turbine cycle with two stages of compression with intercooling. The overall pressure ratio is 9. The air enters each stage of the compressor at 300 K and of the turbine at 1300 K. Utilizing air-standard assumptions, determine the back-work ratio and the thermal efficiency of the cycle. 3. Consider an ideal gas-turbine cycle with two stages of expansion with reheating. The overall pressure ratio is 9. The air the compressor at 300 K and the air enter each stage of the turbine at 1300 K. Utilizing air-standard assumptions, determine the back-work ratio and the thermal efficiency of the cycle.arrow_forwardRequired information Problem 09.052 - Ideal Diesel Cycle with Variable Specific Heats - DEPENDENT MULTI-PART PROBLEM - ASSIGN ALL PARTS An air-standard Diesel cycle has a compression ratio of 16 and a cutoff ratio of 2. At the beginning of the compression process, air is at 103 kPa and 27°C. Account for the variation of specific heats with temperature. The gas constant of air is R= 0.287 kJ/kg-K. Problem 09.052.b - Thermal Efficiency in Variable Heat Capacity Diesel Cycle Determine the thermal efficiency. (You must provide an answer before moving on to the next part.) The thermal efficiency is 59.38 %.arrow_forward2. The compression ratio of an air-standard Otto cycle is 9.5. Prior to the isentropic compressionprocess, the air is at 100 kPa, 35°C, and 600 cm3. The temperature at the end of the isentropicexpansion process is 800 K. Using specific heat values at room temperature, determine (a) thehighest temperature and pressure in the cycle; (b) the amount of heat transferred in, in kJ; (c)the thermal efficiency; and (d) the mean effective pressure.arrow_forward
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY





