
(a)
Interpretation:
It is to be predicted which of the two nucleophiles in the given pair will react faster with
Concept introduction:
The minimum amount of energy required by reacting molecules to reach the transition state is called the activation energy.
For nucleophilic substitution reactions, if the reactant is the same, the activation energy increases with increasing stability of the substitution product.
The reaction is faster when the energy barrier between the reactants and products is small. When energy difference between reactants and the transition state is smaller than the energy difference between products and transition state, the reactants are highly reactive and reaction is faster. Highly reactive reactant molecules serve as strong nucleophiles. The reactants are less reactive and the reaction slower, when the transition state is closer in energy to the products than the reactants.
Answer to Problem 9.4P
(i) Among the two nucleophiles, the nucleophile
(ii) Among the two nucleophiles, the nucleophile
Explanation of Solution
(i) The first given pair of nucleophiles is
The reactions of each of these nucleophiles with
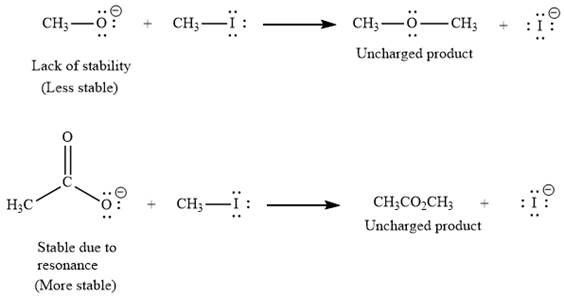
There is a significant difference in charge stability on the reactant side but not on the product side because the negative charge in
(ii) The second given pair of nucleophiles is
The reactions of each of these nucleophiles with
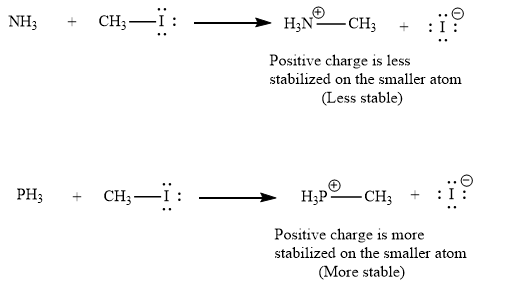
There is a significant difference in charge stability on the product side but not on the reactant side. Because a positive charge is more stable on phosphorous atom than on nitrogen atom, the product of the nucleophilic substitution involving
Therefore, the energy barrier involving
As nucleophilicity of the attacking species increases in
(b)
Interpretation:
It is to be determined which of the given pairs is the stronger nucleophile.
Concept introduction:
The minimum amount of energy required by reacting molecules to reach the transition state is called the activation energy. For nucleophilic substitution reactions, if the reactant is the same, the activation energy increases with increasing stability of the substitution product. When energy difference between reactants and the transition state is smaller than the energy difference between products and transition state, the reactants are highly reactive and reaction is faster. Highly reactive reactant molecules serves as strong nucleophiles.
Answer to Problem 9.4P
(i) Among the pair of nucleophiles,
(ii) Among the two nucleophiles,
Explanation of Solution
(i) The first given pair of nucleophiles is
In part (a), it is determined that the reaction of
(ii) The first given pair of nucleophiles is
In part (a), it is determined that the reaction of
Among the two given nucleophiles, the stronger nucleophile is the one that exhibits a faster reaction.
Want to see more full solutions like this?
Chapter 9 Solutions
Organic Chemistry: Principles and Mechanisms (Second Edition)
- Please draw a curved arrow mechanism for the following reaction. You may use generic acids and bases to protonate and deprotonate when appropriate, but it is in ACIDIC solution. m= & Br₂ H+arrow_forwardProblem: Propose a mechanism for the following transformation. (a) (b) + HBr + H₂O H₂SO4 HO Brarrow_forwardPROBLEM Predict the product(s) and provide the mechanism for each of the 18-28 following reactions. (I PROBLEM Predict the product(s) and provide the mechanism for each of the 18-28 following reactions. (a) (b) H. CH3 HBr ? ether CH3 H30+ ?arrow_forward
- Provide the complete the following mechanism for the reaction below. You must include appropriate arrows,intermediates, and formal charges. Please answer fast i give upvotearrow_forwardCan you help me draw the mechanism arrows for this reaction and determine if the reaction is going forward or reverse?arrow_forwardThe reaction shown here is called the pinacol rearrangement. A carbocation rearrangement is believed to be involved. (a) Propose a reasonable mechanism for this reaction. (b) Suggest why the carbocation rearrangement is favorable. HO ОНarrow_forward
- Use curved arrow formalism to show the mechanism of the reaction shown below.arrow_forwardThe reaction shown here yields three different nucleophilic substitution products that are constitutional isomers of one another. (a) Does this suggest an SN1 or Sn2 mechanism? (b) Draw the mechanism for the formation of each of these products. CH3CH2OH Brarrow_forwardFor the reaction given below, draw a mechanism (curved arrows) and then predict which side of the reaction is favoured under equilibrium conditions.arrow_forward
- Consider the nucleophilic substitution reaction shown here. (a) Argue whether this reaction takes place via an Sn1 or an Sn2 reaction. (b) Draw the complete mechanism (including curved arrows) for this reaction. KI Brarrow_forward↑ ... Propose a reasonable stepwise mechanism, using curved arrow notation to show the flow of electrons, for the following reaction. KCN H EtOH, H₂O OH 02N O₂N NO2 Tt O ọ ♡ > Րarrow_forwardelectrophile the following (Q)Predict the position of affaek for the incoming electrophide on ofisubstituted benzene.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





