
Concept explainers
(a)
Interpretation: The more reactive alkene towards the acid-catalyzed hydration is to be interpreted.
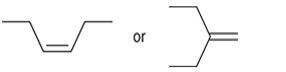
Concept introduction:
(b)
Interpretation: The more reactive alkene from 2-methyl-2-butene and 3-methyl-1-butene toward the acid-catalyzed hydration is to be interpreted.
Concept introduction:
Alkenes are unsaturated hydrocarbons with at least one double bond between the carbon atoms. The presence of pi bonds in these molecules makes them more reactive compared to saturated hydrocarbons; alkanes. The stability of alkenes depends on the substituted groups.
Want to see the full answer?
Check out a sample textbook solution
Chapter 8 Solutions
EBK ORGANIC CHEMISTRY-PRINT COMPANION (
- How many moles of Bra are required to completely halogenate the alkene?A. One moleB. Two molesC. Three molesD. Four moles What is the expected arrangement of the bromine atoms relative to each other amongthe carbon involved in pi bonding?A. anti-conformationB. syn-conformationC. trans-configurationD. cis-configuration What happens to bromine when it is adjacent to an alkene during a chemical reaction?A. Bromine becomes stable. (? kasi before brown siya/acidic tas naging colorless? Jk ewan)B. Bromine becomes polarized.C. Bromine becomes hybridized.D. Bromine becomes acidic. The relative arrangement of bromine atoms in the product is primarily due to:A. ElectronegativityB. RepulsionC. Hydrogen bondingD. Atomic weightWhat is your observation after the reaction?A. A yellow flame is produced.B. Bromine water decolorizes.C. The alkene becomes denser.D. A brown precipitate forms.arrow_forwardWhich of the alkyl chlorides listed below undergoes dehydrohalogenation in the presence of a strong base to give 2-pentene as the only alkene product? Select one: A. 1-chloropentane B. 3-chloropentane C. 2-chloropentane D. 1-chloro-2-methylbutane Which of the following is the most stable species? Select one: CH3 A. о в. H3C CH3 O D.arrow_forward1) Give the major organic product of the following reaction. Don't forget stereochemistry in certain cases. 2) Assign the reaction as addition, elimination, substitution, or rearrangement. A. B. C. D. xx D D 1. H₂O/Hg(OAc)2 2. NaBH₂ Br₂ CCI (inert solvent) H₂/Pd/C HBr Type of Reaction: Type of Reaction: Type of Reaction: Type of Reaction:arrow_forward
- 3. ( Predict the organic product(s) of the reaction of 2-butene with each reagent. a. H₂O (H₂SO4) b. Cl₂ c. Br₂ in H₂Oarrow_forwardFor each reaction identify the reaction type. Reaction Туре a. Methylbenzene and bromine in the presence of ultraviolet light forming 1- bromo-2-methylbenzene and hydrogen bromide. b. 2-methyldecane plus hydrogen reacts to produce heptane and butane c. Hex-2-ene plus hydrogen chloride produces 2-chlorohexane. d. Propane plus pentane reacts to produce 1,2-dimethylcyclohexane and hydrogenarrow_forwardWhat is the major product when 2-iodopentane is used in dehydrohalogenation reaction. A. 2-iodo-1-pentene B. 2-pentene C. (E)-2-pentene D. (Z)-2-pentene E. (Cis)-2-pentenearrow_forward
- Br2 ? How many moles of Br are required to completely halogenate the alkene? A. Three moles B. Four moles C. Two moles D. One mole What is the expected arrangement of the bromine atoms relative to each other among the carbon involved in pi bonding? A. trans-configuration B. anti-conformation C. cis-configuration D. syn-conformation What happens to bromine when it is adjacent to an alkene during a chemical reaction? A. Bromine becomes polarized. B. Bromine becomes acidic. C. Bromine becomes hybridized. D. Bromine becomes stable. The relative arrangement of bromine atoms in the product is primarily due to: A. Hydrogen bonding B. Electronegativity C. Atomic weight D. Repulsion What is your observation after the reaction? A. Bromine water decolorizes. B. A brown precipitate forms. C. The alkene becomes denser. D. A yellow flame is produced.arrow_forward5. Which among the following compounds can show E-Z system. a. 3-bromo-2-chloropent-2-ene b. 3-ethyl-5-methyloct-3-ene c. 3-bromo-2-methylhex-3-enearrow_forward1) Which of the following the alkyl halides reacts the fastest in an SN2 reaction? * A. 1-Chloropropane B. 1-Bromopropane C. 1-Fluoropropane D. 1-Iodopropane E. Alkyl halides do not undergo SN2 reactions 2) The IUPAC name of the expected Markovnikov addition product of HI to 2-methyl-2-butene is __________.arrow_forward
- 5. Give the reagent or chemical test that would differentiate the following pairs of compounds. Provide only the reagents or chemical tests discussed in the module. Write chemical equations for the reactions involved. a. benzene and ethylbenzene b. 1-butyne and 2-butyne c. 2-methylpentane and 2-methyl-2-pentene d. toluene and 1-methylcyclohexene Example on next page.arrow_forwardChoose the two alkyl bromides and the hydrogenation method that must be used to synthesize these alkenes from acetylene. Enter your answer as two letters in alphabetical order, followed by a number; i.e. ac2, not ca2. Do not use punctuation. Alkyl Halides a. CH3CH₂Br b. CH3(CH₂)4Br c. CH3(CH2)sBr d. CH3(CH₂) Br e. CH3(CH₂)7Br f. CH₂(CH₂) Br g. CH3(CH₂)12Br Hydrogenation Method 1. H₂, Lindlar's catalyst 2. Na, NH3 (1) CH3 Alkene #1: H₂C Alkene #2: H₂C CH3arrow_forward1. Fill in the boxes with the missing reagents or major organic products for the alkene reactions. a. HCl b. H2O, H2SO4 с. d. 1. ВН, 2. H2O2, NaOH е. f. CI .CI g. Cl2, H2O h. 1. Н-О, Hg(OАс)2 2. NaBH4, NaOH i. 1. O3 2. Н:0, H-02 j. OH HO.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





