
Concept explainers
(a)
Interpretation:
To identify the given compound as primary, secondary or tertiary alkyl halide and give a common name to the given compound.
Concept introduction:
Alkyl halide refers to those organic compounds that consist of halogen atom as a
In primary alkyl halide, the halogen group is attached to a carbon atom that is connected to two hydrogen atoms and the other carbon atom.
In secondary alkyl halide, the halogen group is attached to a carbon atom that is connected to two carbon atoms and one hydrogen atom.
In tertiary alkyl halide, the halogen group is attached to a carbon atom that is connected to three carbon atoms.
Answer to Problem 8.1P
The common name of the given compound is isobutyl fluoride. It is a primary alkyl halide.
Explanation of Solution
The given compound is
The fluorine atom is attached to a carbon atom that is connected to two hydrogen atoms and the other carbon atom. Therefore, it is a primary alkyl halide.
The common name of the given compound is isobutyl fluoride. It is a primary alkyl halide.
(b)
Interpretation:
To identify the given compound as primary, secondary or tertiary alkyl halide and give a common name to the given compound.
Concept introduction:
Alkyl halide refers to those organic compounds that consist of halogen atom as a functional group attached to the alkyl chain. The general formula is
In primary alkyl halide, the halogen group is attached to a carbon atom that is connected to two hydrogen atoms and the other carbon atom.
In secondary alkyl halide, the halogen group is attached to a carbon atom that is connected to two carbon atoms and one hydrogen atom.
In tertiary alkyl halide, the halogen group is attached to a carbon atom that is connected to three carbon atoms.
Answer to Problem 8.1P
The common name of the given compound is n-hexyl iodide. It is a primary alkyl halide.
Explanation of Solution
The given compound is
The iodine atom is attached to a carbon atom that is connected to two hydrogen atoms and the other carbon atom. Therefore, it is a primary alkyl halide.
The common name of the given compound is n-hexyl iodide. It is a primary alkyl halide.
(c)
Interpretation:
To identify the given compound as primary, secondary or tertiary alkyl halide and give a common name to the given compound.
Concept introduction:
Alkyl halide refers to those organic compounds that consist of halogen atom as a functional group attached to the alkyl chain. The general formula is
In primary alkyl halide, the halogen group is attached to a carbon atom that is connected to two hydrogen atoms and the other carbon atom.
In secondary alkyl halide, the halogen group is attached to a carbon atom that is connected to two carbon atoms and one hydrogen atom.
In tertiary alkyl halide, the halogen group is attached to a carbon atom that is connected to three carbon atoms.
Answer to Problem 8.1P
The common name of the given compound is cyclopentyl bromide. It is a secondary alkyl halide.
Explanation of Solution
The given compound is shown below.
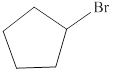
Figure 1
It contains a ring of five carbon atoms. Bromine is attached to the second carbon atom. Therefore, the common name of this compound is cyclopentyl bromide.
The bromine atom is attached to a carbon atom that is connected to two carbon atoms and one hydrogen atom. Therefore, it is a secondary alkyl halide.
The common name of the given compound is cyclopentyl bromide. It is a secondary alkyl halide.
(d)
Interpretation:
To identify the given compound as primary, secondary or tertiary alkyl halide and give a common name to the given compound.
Concept introduction:
Alkyl halide refers to those organic compounds that consist of halogen atom as a functional group attached to the alkyl chain. The general formula is
In primary alkyl halide, the halogen group is attached to a carbon atom that is connected to two hydrogen atoms and the other carbon atom.
In secondary alkyl halide, the halogen group is attached to a carbon atom that is connected to two carbon atoms and one hydrogen atom.
In tertiary alkyl halide, the halogen group is attached to a carbon atom that is connected to three carbon atoms.
Answer to Problem 8.1P
The common name of the given compound is neopentyl chloride. It is a primary alkyl halide.
Explanation of Solution
The given compound is shown below.
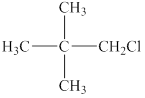
Figure 2
It contains five carbon atoms. Chlorine is attached to the first carbon atom and three methyl groups are attached to the second carbon atom. Therefore, the prefix –neo is used before the name of an alkyl halide. Therefore, the common name of this compound is neopentyl chloride.
The chloride atom is attached to a carbon atom that is connected to two hydrogen atoms and the other carbon atom. Therefore, it is a primary alkyl halide.
The common name of the given compound is neopentyl chloride. It is a primary alkyl halide.
Want to see more full solutions like this?
Chapter 8 Solutions
Organic Chemistry
- (a) Write the IUPAC names of the following compounds :(i) CH3CO(CH2)4CH3 (ii) Ph — CH = CH — CHO(b) Describe the following conversions in not more than two steps :(i) Ethanol to 3-Hydroxybutanal (ii) Benzoic acid to m-Nitrobenzyl alcohol(iii) Propanone to Propenearrow_forwardIn each of the following reactions, two possible organic products can be formed. Draw both organic products in each case and then circle the one formed in greatest quantity in each case. HC (a) 1) NaH, 2) acid (b) CH,CH,OH (c) CH,CH,OH NH2 (d) Oarrow_forward1. (a) Draw structures of the seven isomeric alkynes of formula C6H10 - (b) Give the IUPAC and derived name of each. (c) Indicate which ones will react with Ag' or Cu(NH3)2.arrow_forward
- Write the reagent or draw structures of the starting material or organic product(s) in the following reactions. If more than one product is formed, identify the major product where possible. (a) (b) HO OH OH H2SO4 ? Cl₂ ? FeCl3arrow_forwardA certain hydrocarbon has a molecular formula of C5H8. Which of the following is not a structural possibility for this hydrocarbon: (d) It contains an alkyne O It contains one ring and one double bond (c) It contains two double bonds and no rings O (b) It contains one ring and no double bondsarrow_forward(b) NABH, CH3 COCH,CH3 CH3CH2OH (c)arrow_forward
- Write the bond line formula of the following compounds: (a) 4-methyl-2-hexene, two geometrical (stereoisomers) isomers (b) 3-fluoro-2-methylheptanol (3-fluoro-2-methylheptan-1-ol) (c) 4-methyl-hex-1-yn-3-olarrow_forwardIdentify and classify the functional group in each of the following molecules. (a) CH,CH CCH alkane (b) H3C C-0-CH ester (c) CH,CH2NH2 alkyne (d) H;C CH2 CH, ketone (e) CH,CH OCH,CH, ester OH (f) H,C -CH CH, ester (g) CH,CO,H carboxylic acid v (h) CH,CH,CH=CH2 alcohol (i) H,C alkynearrow_forwardWrite structural formulas for compounds that meet the following descriptions:(a) An alkene, C6H12, that cannot have cis–trans isomersand whose longest chain is 5 carbons long(b) An alkene with a chemical formula of C10H12 that hascis–trans isomers and contains a benzene ring.arrow_forward
- Indicate whether each statement is true or false. (a) Pentanehas a higher molar mass than hexane. (b) The longer the linearalkyl chain for straight-chain hydrocarbons, the higherthe boiling point. (c) The local geometry around the alkynegroup is linear. (d) Propane has two structural isomers.arrow_forwardIndicate whether each statement is true or false. (a) Twogeometric isomers of pentane are n-pentane and neopentane.(b) Alkenes can have cis and trans isomers around theCC double bond. (c) Alkynes can have cis and trans isomersaround the CC triple bond.arrow_forwardWrite Lewis structures and describe the molecular geometry at each carbon atom in the following compounds:(a) cis-3-hexene(b) cis-1-chloro-2-bromoethene(c) 2-pentyne(d) trans-6-ethyl-7-methyl-2-octenearrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





