
Concept explainers
(a)
Interpretation: Check whether the given molecule is polar or not. If the molecule is polar then the direction of polarity has to be indicated.
Concept Introduction:
Polar molecules are the molecules having a positive and negative end and so there will be a charge separation.
A polar molecule is a molecule where the polar bonds are asymmetrically arranged (the dipoles do not cancel)
A nonpolar molecule is a molecule with no polar bonds or a molecule where the polar bonds are symmetrically arranged.
In polar molecule the charge separation occurred with respect to the difference in electronegativity of atoms in the molecule.
Direction of dipole moment in a molecule is can be represented as follows,

Different types of molecules and their geometry in accordance with the VSEPR theory are mentioned below,
(a)
Explanation of Solution
The structure of
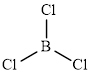
This molecule has a trigonal planar geometry. The direction dipole in the molecule is represented below,
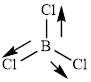
However
(b)
Interpretation: Check whether the given molecule is polar or not. If the molecule is polar then the direction of polarity has to be indicated.
Concept Introduction:
Polar molecules are the molecules having a positive and negative end and so there will be a charge separation.
A polar molecule is a molecule where the polar bonds are asymmetrically arranged (the dipoles do not cancel)
A nonpolar molecule is a molecule with no polar bonds or a molecule where the polar bonds are symmetrically arranged.
In polar molecule the charge separation occurred with respect to the difference in electronegativity of atoms in the molecule.
Direction of dipole moment in a molecule is can be represented as follows,

Different types of molecules and their geometry in accordance with the VSEPR theory are mentioned below,
(b)
Explanation of Solution
The structure of
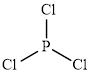
This molecule has a trigonal planar geometry. The direction dipole in the molecule is represented below,
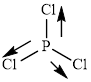
However
(c)
Interpretation: Check whether the given molecule is polar or not. If the molecule is polar then the direction of polarity has to be indicated.
Concept Introduction:
Polar molecules are the molecules having a positive and negative end (dipole) and so there will be a charge separation.
A polar molecule is a molecule where the polar bonds are asymmetrically arranged (the dipoles do not cancel)
A nonpolar molecule is a molecule with no polar bonds or a molecule where the polar bonds are symmetrically arranged.
In polar molecule the charge separation occurred with respect to the difference in electronegativity of atoms in the molecule.
Direction of dipole in a molecule is can be represented as follows,

Different types of molecules and their geometry in accordance with the VSEPR theory are mentioned below,
(c)
Explanation of Solution
The structure of
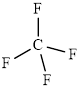
This molecule has a tetrahedral geometry. The direction dipole in the molecule is represented below,
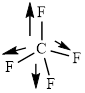
However
(d)
Interpretation: Check whether the given molecule is polar or not. If the molecule is polar then the direction of polarity has to be indicated.
Concept Introduction:
Polar molecules are the molecules having a positive and negative end and so there will be a charge separation.
A polar molecule is a molecule where the polar bonds are asymmetrically arranged (the dipoles do not cancel)
A nonpolar molecule is a molecule with no polar bonds or a molecule where the polar bonds are symmetrically arranged.
In polar molecule the charge separation occurred with respect to the difference in electronegativity of atoms in the molecule.
Direction of dipole moment in a molecule is can be represented as follows,

Different types of molecules and their geometry in accordance with the VSEPR theory are mentioned below,
(d)
Explanation of Solution
The structure of
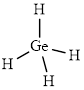
This molecule has a tetrahedral geometry. The direction dipole in the molecule is represented below,
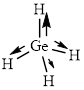
However
Want to see more full solutions like this?
Chapter 8 Solutions
Chemistry & Chemical Reactivity
- Given the bonds C N, C H, C Br, and S O, (a) which atom in each is the more electronegative? (b) which of these bonds is the most polar?arrow_forwardWhich statement is always true according to VSEPR theory?(a) The shape of a molecule is determined only by repulsions among bonding electron groups.(b) The shape of a molecule is determined only by repulsions among nonbonding electron groups.(c) The shape of a molecule is determined by the polarity of its bonds.(d) The shape of a molecule is determined by repulsions among all electron groups on the central atom (or interior atoms, if there is more than one).arrow_forwardUrea, (NH2)2CO, is used in plastics and fertilizers. It is also the primary nitrogencontaining substance excreted by humans.(a) Which bonds in the molecule are polar, and which are nonpolar?(b) Which is the most polar bond in the molecule? Which atom is the negative end of the bond dipole?arrow_forward
- Which of the following statements is correct? (A) Only neutral molecules can be non-polar, all ions are polar. (B) The net dipole moment of non-polar molecules/ions is 0. (C) All linear molecules are non-polar. (D) All molecules that contains only polar bonds are polar.arrow_forwardWhich of these molecules and ions contain polar bonds? Which of these molecules and ions have dipolemoments?(a) H3O+(b) PCl4−(c) SnCl3−(d) BrCl4−(e) ICl3(f) XeF4(g) SF2arrow_forwardConsider the molecules BF₃, PF₃, BrF₃, SF₄, and SF₆.(a) Which has bonds that are the most polar?(b) Which have a molecular dipole moment?arrow_forward
- Which compounds have nonpolar covalent bonds, which have polar covalent bonds, and which have ions? (a) LiF (b) CH3F (c) MgCl2 (d) HClarrow_forward3) The molecule diphosphorus tetraoxide (P,O,) has two central atoms and four different resonance structures that do not violate the octet rule. Draw two of these resonance structures below. 4) The compound acetone is a common solvent. It has a chemical formula of CH,COCH, Acetone has three central atoms. (a) Draw the Lewis Dot structure for acetone. (b) Give the Ideal Bond Angle for all three central atoms. 5) Four covalent molecules are drawn below. :o: H. H-CH H H (1) (2) (3) (4) a) Define each of these molecules as polar or non-polar. (1) (2) (3) b) Describe the type of intermolecular force that each molecule would use: (1) (2) (3) (4)arrow_forwardAn elemental analysis of a hydrocarbon, which contains only carbon and hydrogen, shows the mass%: element mass% carbon 92.26 hydrogen 7.743 (A) * Determine the empirical formula of the hydrocarbon. (B) The compound has a molar mass of 26.04 g/mol. Determine its molecular formula. (C, Draw the Lewis structure of the molecular compound. Count the total number of sigma bonds and pi bonds each, present in the molecule. (D, What is the hybridization of carbon in the molecule? Explain.arrow_forward
- 1. Draw the Lewis structures for each of the following ions or molecules. For each, give (i) the molecular shape, (ii) the electron pair geometry at the central atom, and (iii) the hybridization of the central atom. (a) POF3 (b) XeO₂F3+ (c) BrCl₂ (d) N3 (the central atom is N; two other N's are bonded to it) (e) PF3arrow_forwardWhich of the following molecules have dipole moments?(a) CS2(b) SeS2(c) CCl2F2(d) PCl3 (P is the central atom)(e) ClNO (N is the central atom)arrow_forwardWhich of the following molecules have dipole moments? (a) CS2 (b) SeS2 (c) CCl2F2 (d) PCl3 (P is the central atom) (e) ClNO (N is the central atom)arrow_forward
 World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning


