
Concept explainers
(a)
Interpretation:
The structure and the stereochemistry of products formed by the reaction,
Concept introduction:
The addition of
Answer to Problem 7.55AP
The products formed by the reaction,
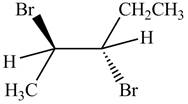
The stereo isomeric products that are formed are in the same amounts. Therefore, the racemic mixture is obtained.
Explanation of Solution
The reaction of
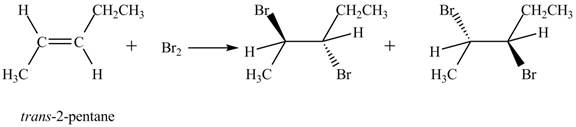
Figure 1
The products obtained are in equal ratio. Therefore, the racemic mixture is obtained.
The products formed by the reaction
(b)
Interpretation:
The structure and the stereochemistry of products formed by the reaction,
Concept introduction:
The addition of
Answer to Problem 7.55AP
The products formed by the reaction
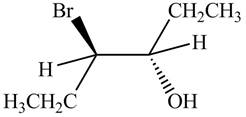
The stereo isomeric products that are formed are in the same amounts. Therefore, the racemic mixture is obtained.
Explanation of Solution
The reaction of
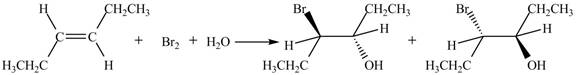
Figure 2
The products obtained are in equal ratio. Therefore, the racemic mixture is obtained.
The products formed by the reaction
(c)
Interpretation:
The structure and the stereochemistry of products formed by the reaction
Concept introduction:
The addition of
Answer to Problem 7.55AP
The products formed by the reaction
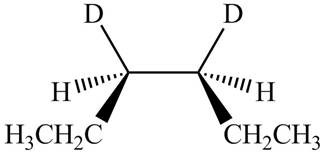
The stereoisomeric product that is obtained by the
Explanation of Solution
The reaction of
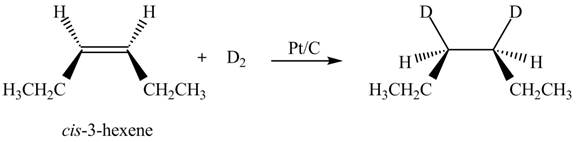
Figure 3
Therefore, the compound obtained is meso compound.
The products formed by the reaction
The stereo isomeric product that is obtained by the
(d)
Interpretation:
The structure and the stereochemistry of products formed by the reaction
Concept introduction:
The addition of
Answer to Problem 7.55AP
The product formed by the reaction,
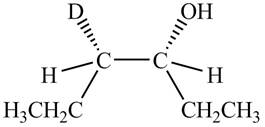
The stereo isomeric products that are formed are in the same amounts. Therefore, the racemic mixture is obtained.
Explanation of Solution
The reaction of
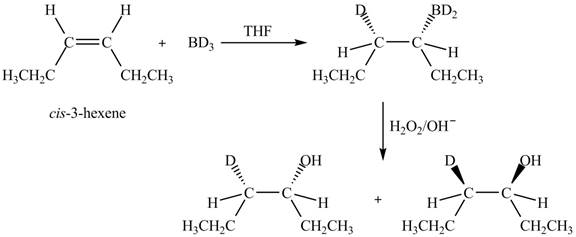
Figure 4
The products obtained are in equal ratio. Therefore, the racemic mixture is obtained.
The products formed by the reaction
The stereo isomeric products that are formed are in the same amounts.
Want to see more full solutions like this?
Chapter 7 Solutions
Organic Chemistry
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





