
Interpretation:
The compound with the molecular formula
Concept introduction:
>The
In dehydrohalogenation
The elimination of primary alkyl halides proceeds through
In
According to Zaitsev rule, the most substituted alkene is the major product.
Answer to Problem 43P
Solution:
The compounds of molecular formula
a)
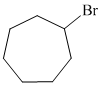
b)
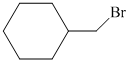
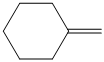
c)
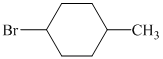
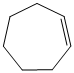
d)
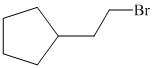
e)
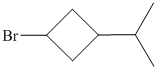
f)
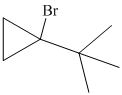
Explanation of Solution
a) The structure of the given alkene as the product of
In
Hence, the structure of alkyl bromide is as follows:

b) The structure of the given alkene as the product of
In
So, there are two possible structures of alkyl bromide which are as follows:
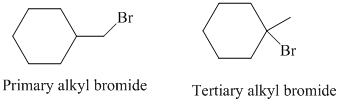
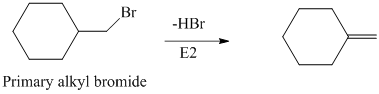
The primary alkyl bromide forms the desired product but the tertiary alkyl bromide can form two alkenes with trisubstituted alkene as the major product. The reaction is as follows:
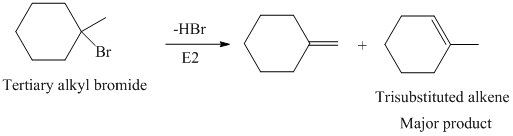
Hence, the structure of the compound that forms the given alkene as an exclusive product of
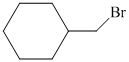
c)The structure of given alkene as the product of
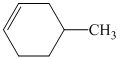
In
So, there are two possible structures of alkyl bromide as follows:
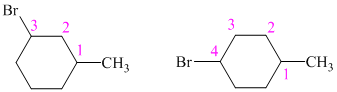
The alkyl bromide in which
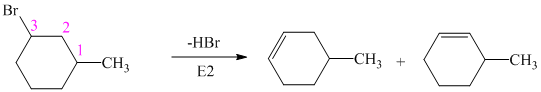
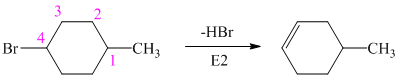
Hence, the structure of the compound that forms the given alkene as an exclusive product of

d) The structure of the given alkene as the product of
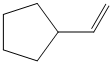
In
So, there are two possible structures of alkyl bromide as follows:
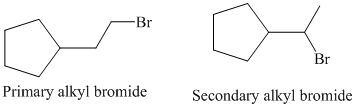
The primary alkyl bromide forms the desired product but the secondary alkyl bromide can form two alkenes with trisubstituted alkene as the major product.
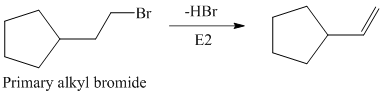
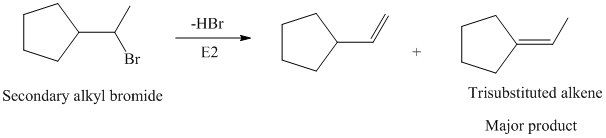
Hence, the structure of the compound that forms the given alkene as an exclusive product of

e) The structural formula of the given alkene is shown below:
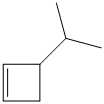
In
So, there are two possible structures of alkyl bromide as follows:
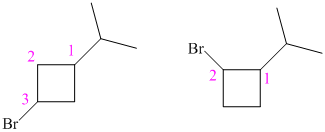
The alkyl bromide in which
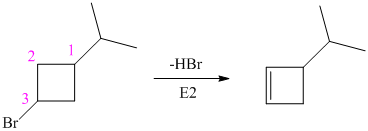
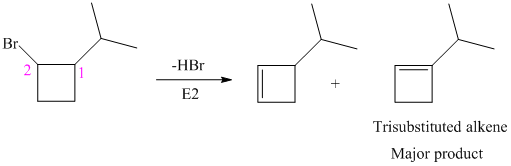
Hence, the structure of the compound that forms the given alkene as an exclusive product of

f) The structural formula of the given alkene is shown below:
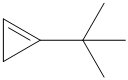
In
So, there are two possible structures of alkyl bromide as follows:
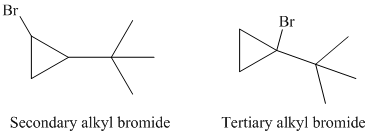
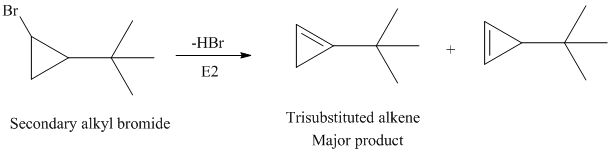
The secondary alkyl bromide forms two alkenesbut the tertiary alkyl bromide forms only the desired product as shown in following equations:
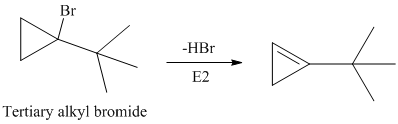
Hence, the structure of the compound that forms the given alkene as an exclusive product of

Want to see more full solutions like this?
Chapter 7 Solutions
Organic Chemistry - Standalone book
- Dehydration of 1,2,2-trimethylcyclohexanol with H2SO4 affords 1-tert-butylcyclopentene as a minor product. (a) Draw a stepwise mechanism that shows how this alkene is formed. (b) Draw other alkenes formed in this dehydration. At least one must contain a five-membered ring.arrow_forwarda) Draw two different enol tautomers of 2-methylcyclohexanone. (b) Draw two constitutional isomers that are not tautomers, but contain a C = C and an OH group.arrow_forwardDraw the organic products formed when cyclopentene is treated withfollowing reagent. [1] CH3CO3H; [2] H2O, HO−arrow_forward
- c) Classify the reaction below as an oxidation, a reduction, or neither.cis-pent-2-ene → pentaneA) oxidationB) reductionC) neitherAnswer: 2) Which of the following compounds has carbon in the highest oxidation state?A) CO2B) CH3COCH3C) CH3CH2OHD) CH3C(OCH2CH3)3Answer: 3) Which of the following reagents is the best choice for oxidizing a primary alcohol toan aldehyde?A) H2CrO4B) pyridinium chlorochromateC) Na2Cr2O7, H2SO4D) KMnO4E) LiAlH4Answer:arrow_forward1) Draw the structures of the following compounds from the a) 5,6,7R-trimethyl-2E,5Z-nonadiene b) 4R-ethyl-3S-methylcyclohexene.arrow_forward1) Draw the structure of the alkene product for the E2 elimination reaction shown below. 1)a 2)b 3)c 4)d 5)earrow_forward
- 2) Give the reagents to make each product from the starting molecule. a) CH,=CHCH,CH,CH, b) c) Brarrow_forwardA is a toxin produced by the poisonous seaweed Chlorodesmis fastigiata. (a) Label each alkene that exhibits stereoisomerism as E or Z. (b) Draw a stereoisomer of A that has all Z double bonds.arrow_forwardFor alkenes A, B, and C: (a) Rank A, B, and C in order of increasing heat of hydrogenation; (b) rank A, B, and C in order of increasing rate of reaction with H2, Pd-C; (c) draw the products formed when each alkene is treated with ozone, followed by Zn, H2O.arrow_forward
- Draw a structure for the product of nucleophilic substitution obtained on solvolysis of tert-butyl bromide in methanol, and arrange the correct mechanism for its formation. Be sure to answer all parts.arrow_forwardThe addition reaction of an acid (HBr) to an alkene (CH3CH=CH2) follows Markovnikov's rule and involves: A) initial attack by Br– B) initial attack by Br• C) isomerization of CH3CH2CH2Br D) formation of a primary carbocation. E) formation of a secondary carbonation. (F) Formation of allyl carbocationarrow_forwardFor alkenes A, B, C, and D: (a) Rank A—D in order of increasing heat of hydrogenation; (b) rank A—D in order of increasing rate of reaction with H2, Pd-C; (c) draw the products formed when each alkene is treated with ozone, followed by Zn, H2O.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





