
Concept explainers
Consider the following proposed structures for benzene, each of which is consistent with the molecular formula
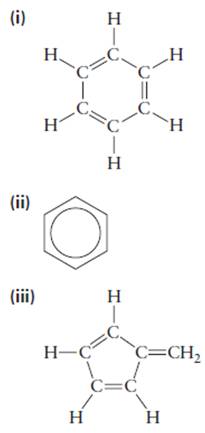
(iv)
(v)
When benzene reacts with chlorine to give
- only one isomer of that compound forms. Which of the five proposed structures for benzene are consistent with this observation? When
Want to see the full answer?
Check out a sample textbook solution
Chapter 7 Solutions
Principles of Modern Chemistry
- The following is a structural diagram for penicillin G, an antibiotic compound with outstanding antibacterial activity. It is obtained from the liquid filtrate of molds. HO H H H C-C N- H Penicillin G can be described as an Organic Co -N- organic inorganic CH₂ H CH₂ -COOH compound. One functional group found in penicillin G is the ◆ grouparrow_forwardTRUE OR FALSE (a) A functional group is a group of atoms in an organic molecule that undergoes a predictable set of chemical reactions. (b) The functional group of an alcohol, an aldehyde, and a ketone have in common the fact that each contains a single oxygen atom. (c) A primary alcohol has one —OH group, a secondary alcohol has two —OH groups, and a tertiary alcohol has three —OH groups. (d) There are two alcohols with the molecular formula C3H8O. (e) There are three amines with the molecular formula C3H9N. (f) Aldehydes, ketones, carboxylic acids, and esters all contain a carbonyl group. (g) A compound with the molecular formula of C3H6O may be either an aldehyde, a ketone, or a carboxylic acid. (h) Bond angles about the carbonyl carbon of an aldehyde, a ketone, a carboxylic acid, and an ester are all approximately 109.5°. (i) The molecular formula of the smallest aldehyde is C3H6O, and that of the smallest ketone is also C3H6O. (j) The molecular formula of the smallest carboxylic…arrow_forwardDraw structures that fit each description and name the functional group in each molecule: (a) two constitutional isomers with molecular formula C5H10O that contain different functional groups; (b) two constitutional isomers with molecular formula C6H10O that contain the same functional group.arrow_forward
- A certain hydrocarbon has a molecular formula of C5H8. Which of the following is not a structural possibility for this hydrocarbon? (a) It is a cycloalkane. (b) It contains one ring and one double bond. (c) It contains two double bonds and no rings. (d) It is an alkyne.arrow_forwardDraw the structural formulas for the following compounds. Include all the bonds to hydrogen atoms. Be sure to answer both parts. (a) 1,4-dichloro-2-ethylbenzene: (b) 2-ethyl-1,3-dimethylbenzene:arrow_forwardThis question is about the chemistry of alkenes, which are unsaturated hydrocarbons. (a) State what is meant by the term unsaturated as applied to a hydrocarbon. (1) (b) An organic compound, X, is an unsaturated hydrocarbon with molecular formula CH₂. (i) Draw the displayed formulae and give the names of two molecules with molecular formula C₂H, which are E/Z isomers. (3) Isomer 1 Isomer 2 Name: Name:arrow_forward
- TRUE OR FALSE (a) Both ethylene and acetylene are planar molecules. (b) An alkene in which each carbon of the double bond has two different groups bonded to it will show cis-trans isomerism. (c) Cis-trans isomers have the same molecular formula but a different connectivity of their atoms. (d) Cis-2-butene and trans -2-butene can be interconverted by rotation about the carbon–carbon double bond. (e) Cis-trans isomerism is possible only among appropriately substituted alkenes. (f) Both 2-hexene and 3-hexene can exist as pairs of cis-trans isomers. (g) Cyclohexene can exist as a pair of cis-trans isomers. (h) 1-Chloropropene can exist as a pair of cis-trans isomers.arrow_forwardDraw skeletal structures for the cyclopropane (three-membered ring) isomers with a formula of C₅H₁₀. Note: cyclopropane is a carbon-carbon ring with three carbons. Three isomersarrow_forwardwrite the structure formulas of alkanes with molecular formula C6H14, which with chlorine give: a) three monochlorinated isomers? b) five monochlorinated isomers c) only two monochlorinated isomersarrow_forward
- Glucose, C6H12O6, contains an aldehyde group but exist predominantly in the form of the cyclic hemiacetal show below. A cyclic hemiacetal is formed when the —OH group of one carbon bonds to the carbonyl group of another carbon. Identify which carbon provides the —OH group and which provides the —CHO? Give a functional isomer of glucose and draw its structure.arrow_forwardDraw skeletal structures for the cyclopropane (three- membered ring) isomers with a formula of C5H10. Note: cyclopropane is a carbon-carbon ring with three carbons.arrow_forwardCan a hydrocarbon have each of the following molecular formulas? Explain why or why not in each case. (a) C 3H 8; (b) C 3H 9; (c) C 3H 6arrow_forward
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning


