
Concept explainers
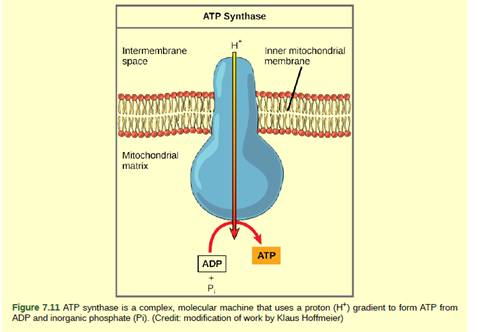
Figure 7.11 Dinitrophenol (DNP) is an "uncoupler" that makes the inner mitochondrial membrane "leaky" to protons. It was used until 1938 as a weight-loss drug. What effect would you expect DNP to have on the change in pH across the inner mitochondrial membrane? Why do you think this might be an effective weight-loss drug?
To write:
The effect of DNP on the pH across the mitochondrial membrane and the reason that it is a weight-loss drug.
Introduction:
Dinitrophenol (DNP) is an uncoupler. The flow of electrons can be separated by DNP along with the pumping of the H+ ion for the purpose of production of ATP (adenosine triphosphate). It means that the electron transport chain can no longer form a proton gradient, and ATP synthase can no longer make ATP.
DNP is a drug given to the patient for losing weight. After using DNA as weight loss drug, a person obtains less energy out of the eaten food. One of the worst side effects of taking DNP is overheating of the body, the energy from electron transport is lost as heat.
Explanation of Solution
DNP is an uncoupler, it disrupts the ATP synthesis by leaking protons across the inner mitochondrial membrane. As a result, the proton gradient cannot be formed across the inner mitochondrial membrane as pumping of H+ (hydrogen ion) is stopped. So, there will be a decrease in pH across the inner membrane of mitochondria.
Therefore, ATP production becoming less efficient. Hence, the energy which is normally produced during cellular respiration is being wasted as heat. In such condition, majority of the eaten food could not be used for the purpose of ATP synthesis and we lose weight.
DNP is an effective diet drug used to lose weight. It acts as an uncoupler which disrupts the H+ gradient across mitochondrial membrane reducing ATP synthesis. Since ATP cannot be formed, the energy from electron transport is lost as heat.
Want to see more full solutions like this?
Chapter 7 Solutions
Biology 2e
Additional Science Textbook Solutions
College Physics
Campbell Biology in Focus
Concepts of Genetics (11th Edition)
Concepts of Genetics (12th Edition)
Human Physiology: An Integrated Approach (7th Edition)
Campbell Essential Biology with Physiology (6th Edition)
- ATP is synthesized from ADP, P, and a proton on the matrix side of the inner mitochondrial membrane. We will refer to the matrix side as the "inside" of the Inner Mitochondrial Membrane (the "IMM"). Show your work. (a) H* transport from the outside of the IMM into the matrix drives this process. Assume the pH inside the matrix is 8.00 and the outside is more acidic by 0.60 pH units. Assuming the IMM membrane potential is 165.0 mV (inside negative), calculate AG for the transport of 1 mol of H* across the IMM into the matrix at 37 °C: H* (outside) → H* (inside)arrow_forwardEthylene glycol (HO—CH2—CH2—OH) is frequently used as antifreeze in automobile engines. Every year children and pets are poisoned because they tasted this sweet-tasting material. Ethylene glycol is metabolized in the liver by alcohol dehydrogenase. Suggest a possible medical treatment for ethylene glycol intoxication.arrow_forwardDCCD (diocyclohexylcarbodiimide) inhibits oxidative phosphorylation when the substrate is mitochondrial NADH. DCCD is a drug that binds to ATP synthase and blocks proton transport through the ion channel. a) Explain what the consequences of DCCD on cellular energy production are. b) Suggest at least one other cellular effect of DCCD and explain this effect.arrow_forward
- Ethylene glycol (HO−CH2−CH2−OH) is a major component of antifreeze. In the body, it is first converted to HOOC−CHO (oxoethanoic acid) and then to HOOC−COOH (oxalic acid), which is toxic. What class of enzyme catalyzes both of the reactions of ethylene glycol? The treatment for the ingestion of ethylene glycol is an intravenous solution of ethanol. How might this help prevent toxic levels of oxalic acid in the body?arrow_forwardThe reaction pictured is an oxidation-reduction reaction in the citric acid cycle in which the energy-carrier molecule NADH is generated. Identify which molecule in the reaction will be oxidized and which molecule will be reduced. Place a single answer choice in each box. COO- HO-C-H H-C-H COO- Malate NAD+ NADH + H+ Oxidized malate oxaloacetate COO- H-C-H ī COO- Oxaloacetate Reduced NADH NAD+arrow_forwardA student is trying to determine the mechanism for a reaction that uses ATP to activate a carboxylate ion, which then reacts with a thiol. If the carboxyl-ate ion attacks the g-phosphorus of ATP, the reaction products are the thioester, ADP, and phosphate. However, whether it attacks the a-phosphorus or the b-phosphorus of ATP cannot be determined from the reaction products because the thioester, AMP, and pyrophosphate would be the products in both reactions. The mechanisms can be distinguished by a labeling experiment in which the enzyme, the carboxylate ion, ATP, and radioactively labeled pyro-phosphate are incubated, and then the ATP is isolated. If the isolated ATP is radioactive, attack occurred on the a-phosphorus. If it is not radioactive, then attack occurred on the b-phosphorus. Explain these conclusions.arrow_forward
- Which one of the following statements about coenzyme Q is not true? a. coenzyme Q is lipid-soluble b. coenzyme Q can accept one or two electrons c. conenzyme Q often diffuses in the membrane from one protein complex to another in its semiquinone form d. Coenzyme Q is one of the five electron carriers used in the electron transport chainarrow_forwardWhy is the enzyme-catalyzed introduction of carbon–carbon double bonds into fatty acids called an electron transport system?arrow_forwardThe phosphorylation of glucose to glucose 1-phosphate requires 5.0 kcal/mol of energy. This unfavorable reaction can be driven by the hydrolysis of ATP to ADP. (a) Write the equation for the coupled reaction. (b) How much energy is released in the coupled reaction? glucose + HPO4---------->2– glucose 1-phosphate + H2Oarrow_forward
- Imagine that you are studying the metabolism of a baby who is feeding on breast milk. You are going to compare the catabolism of two molecules of galactose from lactose in milk with the catabolism of two molecules of leucine from casein in milk. Detail the catabolic path for the molecules until their final oxidation in CO2 + urea.arrow_forwardThiamine is the vitamin precursor for a co-enzyme called thiamine pyrophosphate or TPP. A patient diagnosed with thiamine deficiency exhibited fatigue and muscle cramps. The muscle cramps have been related to the accumulation of specific metabolic acids. On the following list, circle the metabolic acids that are most likely to accumulate in a thiamine deficiency? Note: There may be more than one. Isocitrate Pyruvate Succinate α-ketoglutarate Malate Fumarate (b) Provide a brief explanation for your answer to 1 (a) herearrow_forwardThe oxidation of 1 mol of glucose supplies enough metabolic energy to form 36 mol of ATP. Oxidation of 1 mol of a typical dietary fat like tristearin (C57H116O6) yields enough energy toform 458 mol of ATP. How many molecules of ATP can form per gram of (a) glucose; (b) tristearin?arrow_forward
 Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax
Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax
