
Concept explainers
MATHEMATICAL The enzyme
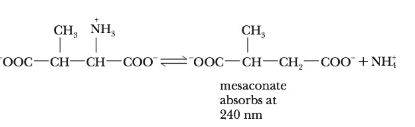
[V. Williams and J. Selbin, J. Biol. Chem. 239, 1636 (1964)]. The rate of the reaction was determined by monitoring the absorbance of the product at 240 nm
Interpretation:
The
Concept introduction:
In an enzymatic reaction,
To determine
To draw this plot, substrate concentration
The
Answer to Problem 28RE
Plotting the data gives the following straight line:
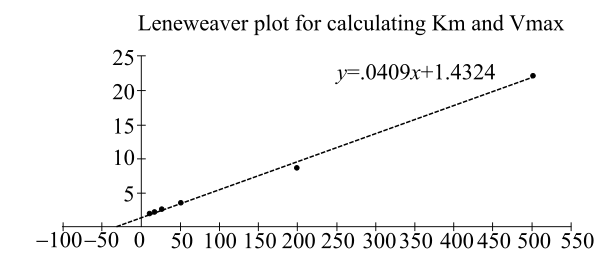
From this straight-line graph, the value of
Explanation of Solution
Given information:
Following data containing substrate concentrations and velocity of the reaction at that substrate concentration, is given.
In enzyme kinetics, to determine
But
Modification of the equation is
This equation can be plotted on the graph,
The enzyme, b-methyl aspartate, catalyzes the deamination of b-methyl aspartate. By plotting the given data into a Lineweaver–Burk plot, Km for this reaction is 2.86 × 10-2 mole. Although in this case, concentration cannot be determined directly. Absorbance values were used instead, as a matter of convenience.
Want to see more full solutions like this?
Chapter 6 Solutions
Biochemistry
- MATHEMATICAL If a reaction can be written AB, and the G is 20kJmol1, what would the substrate/product ratio have to be for the reaction to be thermodynamically favorable?arrow_forwardMATHEMATICAL Consider the reaction AB+C, where G=0.00. (a) What is the value of G (not G) when the initial concentrations of A, B, and C are 1 M, 103M,and106M? (b) Try the same calculations for the reaction D+EF, for the same relative order of concentrations. (c) Try the same calculations for the reaction GH, if the concentrations are 1Mand103M for G and H, respectively.arrow_forwardMATHEMATICAL Determine the values of KM and Vmax for the decarboxylation of a -keto acid given the following data. substrateconcentration(molL1)Velocity(mMmin1)2.5001.0000.7140.5260.2500.5880.5000.4170.3700.256arrow_forward
- MATHEMATICAL For the following aspartase reaction (see Question 28) in the presence of the inhibitor hydroxymethylaspartate, determine KM and whether the inhibition is competitive or noncompetitive. [s](molarity)V,noInhibitor(arbitraryunits)V,InhibitorPresent(samearbitraryunits)110451041.5103510311.00.0260.0920.1360.1659.520.0100.0400.0860.1427.60arrow_forwardREFLECT AND APPLY Why is the development of catalysis important to the development of life?arrow_forwardMATHEMATICAL Calculate the ATP yield for the complete oxidation of one molecule of palmitic acid (16 carbons). How does this figure differ from that obtained for stearic acid (18 carbons)?arrow_forward
- MATHEMATICAL The G for the reaction Citrate Isocitrare is +6.64kJmol1=+1.59kcalmol1. The G for the reaction Isocitrate -Ketoglutrate is 267kJmol1=63.9kcalmol1. What is the G for the conversion of citrate to - ketoglutarate? Is that reaction exergonic or endergonic, and why?arrow_forwardREFLECT AND APPLY The enzyme lactate dehydrogenase catalyzes the reaction Pyruvate+NADH+H+lactate+NAD+ NADH absorbs light at 340 nm in the near-ultraviolet region of the electromagnetic spectrum, but NAD1 does not. Suggest an experimental method for following the rate of this reaction, assuming that you have available a spectrophotometer capable of measuring light at this wavelength.arrow_forwardMATHEMATICAL For the Vmax obtained in Question 26, calculate the turnover number (catalytic rate constant) assuming that 131024mol of enzyme were used.arrow_forward
- REFLECT AND APPLLY Show how the estimate of 33% efficiency of energy use in anaerobic glycolysis is derived.arrow_forwardREFLECT AND APPLY When we compare the binding of I and of S to the enzyme in a mixed noncompetitive inhibitor, we assumed that the binding of I decreased the affinity of the enzyme for S. What would happen if the opposite were true?arrow_forwardMATHEMATICAL What is the ratio of HEPES/HEPES-H+ in a HEPES buffer at pH 7.9?arrow_forward
 BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
