
Concept explainers
Interpretation:
The structure of the major organic product obtained from the reaction of
Concept introduction:
Primary
The solvents are chosen in such a way that both alkyl halide and ionic salt (nucleophile) form a homogenous solution.
In the transition state, both alkyl halide and nucleophile are involved. In the transition state, the carbon is partially bonded with both the incoming nucleophile and the departing halide. As the reaction progresses, the arrangement of bonds undergoes tetrahedral inversion.
Answer to Problem 19P
Solution:
a)
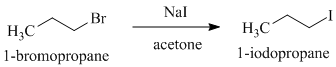
b)
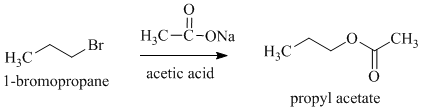
c)
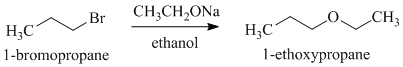
d)
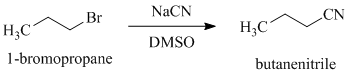
e)
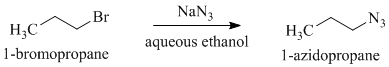
f)
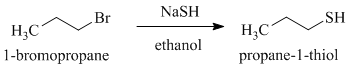
g)
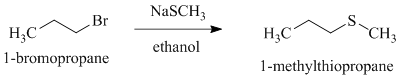
Explanation of Solution
a)

b)

c)
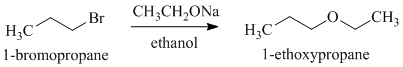
d)

e)

f)
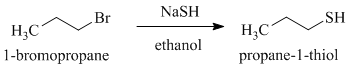
g)
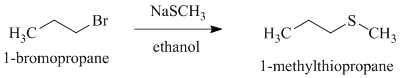
Want to see more full solutions like this?
Chapter 6 Solutions
Organic Chemistry - Standalone book
- When 2-chloropropane treated with NaOH and 1-chloropropane treated with NaOH separately produce two different functional groups. Provide both reactions and explain the two different functional groups produced.arrow_forwardWhat reactions and reagents can be used to make phenol from benzene if electrophilic aromatic substitution reactions are excluded and benzene is the only source of carbon?arrow_forwardWrite structural formulas for all aldehydes with the molecular formula C6H12O and give each its IUPAC name. Which of these aldehydes are chiralarrow_forward
- The ketone 2-heptanone has been identified as contributing to the odor of a number of dairy products, including condensed milk and cheddar cheese. Describe the synthesis of 2-heptanone from acetylene and any necessary organic and inorganic reagents.arrow_forwardGive structures and names of the products, if any, expected from the reaction of isobutylene with: Hydrogen in the presence of finely divided nickel Bromine Hydrogen bromide in the presence of hydrogen peroxide Hydrogen chloride in the presence of hydrogen peroxide Diborane, hydrogen peroxide, sodium hydroxidearrow_forwardHydration of 2-pentyne gives a mixture of two ketones, each with the molecular formula C5H10O. Propose structural formulas for these two ketones and for the enol from which each is derivedarrow_forward
- State the Reactions of Aldehydes and Ketones with Grignard Reagents:arrow_forwardDraw the structure of each product from the reaction of benzene with 2-chloro-1-methylcyclohexane using AlCl 3 as the catalyst and Identify the major product.arrow_forwardWhat is the structure of the principal organic product formed in the reaction of 1-iodopropane with NaOH?arrow_forward
- Which of the following sequences of reactions would convert toluene to 2-bromo-4-cyanotoluene? Bromination, nitration, reduction, diazotization, reaction with cyanide anion Bromination, nitration, diazotization, reduction , reaction with cyanide anion Nitration, bromination, reduction, diazotization, reaction with cyanide anion Nitration, bromination, diazotization, reduction, reaction with cyanide anionarrow_forwardDraw the structural formula of the product of (a) p-methoxybenzaldehyde with each of the following, then do it for (b) 1-phenyl-1-pentanone for each of the following:arrow_forwardExplains the general concept of the manufacture of benzene by dealkylation of toluene with hydrogen.arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning


