
Concept explainers
(a)
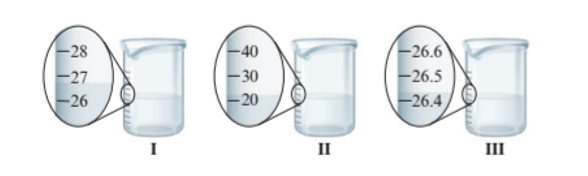
Interpretation : The volume of water in each beaker with the proper number of significant figures needs to be determined.
Concept Introduction : A number can be changed to given significant figure either by round off or by addition of zero. The rules for significant figures are:
- Non-zero digits are always significant.
- Any zeros between two significant digits are always significant.
- A final zero in the decimal portion is also significant.
(a)
Answer to Problem 9STP
Beaker I = 27.0 mL
Beaker II = 20.0 mL
Beaker III = 26.4 mL
Explanation of Solution
Each beaker has its marking of volume. The beaker I and II have the difference of 1 unit of volume whereas beaker III has more precise value of volume as it has difference of 0.1 unit of volume. Thus, the volume of water in each beaker is:
- Beaker I = 27.0 mL
- Beaker II = 20.0 mL
- Beaker III = 26.4 mL
(b)
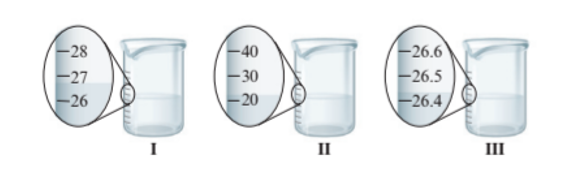
Interpretation : The digits in each measurement from part A needs to be identified as certain and uncertain.
Concept Introduction : A number can be changed to given significant figure either by round off or by addition of zero. The rules for significant figures are:
- Non-zero digits are always significant.
- Any zeros between two significant digits are always significant.
- A final zero in the decimal portion is also significant.
(b)
Answer to Problem 9STP
Beaker I = Uncertain
Beaker II = Uncertain
Beaker III = Certain
Explanation of Solution
The beaker I and II have the difference of 1 unit of volume whereas beaker III has more precise value of volume as it has difference of 0.1 unit of volume. Since beaker III has more precise volume so it has certain value of volume whereas beaker I and II has uncertain value of volume.
(c)
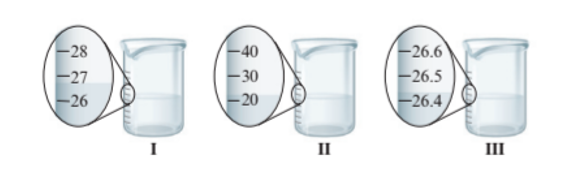
Interpretation : The total volume of water if all the three beakers pour in one container needs to be determined with correct number of significant figures.
Concept Introduction : A number can be changed to given significant figure either by round off or by addition of zero. The rules for significant figures are:
- Non-zero digits are always significant.
- Any zeros between two significant digits are always significant.
- A final zero in the decimal portion is also significant.
(c)
Answer to Problem 9STP
The total volume must be 27.0 +20.0 + 26.4 = 73.4 mL
Explanation of Solution
The beaker I and II have the difference of 1 unit of volume whereas beaker III has more precise value of volume as it has difference of 0.1 unit of volume. The volume of water in each beaker is:
- Beaker I = 27.0 mL
- Beaker II = 20.0 mL
- Beaker III = 26.4 mL
Thus, the total volume must be 27.0 +20.0 + 26.4 = 73.4 mL
Chapter 5 Solutions
World of Chemistry, 3rd edition
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





