
Concept explainers
(a)
Interpretation:
The given molecule is same or different enantiomer of the shown original molecule is to be identified.
Concept introduction:
Isomers are molecules having same connectivity. Enantiomers are nonsuperimposable mirror images. If the molecules can be interconverted by one or more single bond rotation, then they are said to be the same enantiomer or identical molecules. If the molecules are not able to interconvert, then they are enantiomers of each other.
Answer to Problem 5.37P
The given molecule is not same enantiomer as the original one.
Explanation of Solution
The given original enantiomer is:
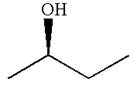
The molecule which is to be compared is:
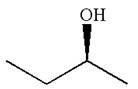
The given molecule is the non superimposable mirror image of THE original molecule.
These molecules cannot be interconverted by single bond rotation. Hence, the given molecule is not same enantiomer as the original one.
The molecule is not a same enantiomer as the original molecule is determined on the basis of capability of interconversion by single bond rotation.
(b)
Interpretation:
The given molecule is same or different enantiomer of the shown original molecule is to be identified.
Concept introduction:
Isomers are the molecules having same connectivity. Enantiomers are nonsuperimposable mirror images. If the molecules can be interconverted by one or more single bond rotation, then they are said to be same enantiomer or identical molecules. If the molecules are not able to interconvert, then they are enantiomers of each other.
Answer to Problem 5.37P
The given molecule is a same enantiomer as the original one.
Explanation of Solution
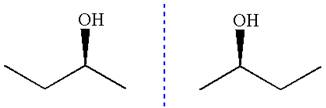
The given original enantiomer is:
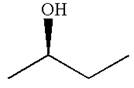
The molecule which is to be compared is:
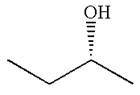
The molecule can be converted to original molecule.
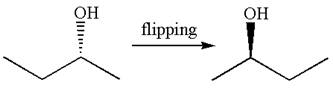
Hence, the given molecule is a same enantiomer as the original one.
The molecule is a same enantiomer as the original molecule is determined on the basis of capability of interconversion by single bond rotation.
(c)
Interpretation:
The given molecule is same or different enantiomer of the shown original molecule is to be identified.
Concept introduction:
Isomers are the molecules having same connectivity. Enantiomers are nonsuperimposable mirror images. If the molecules can be interconverted by one or more single bond rotation, then they are said to be the same enantiomer or identical molecules. If the molecules are not able to interconvert, then they are enantiomers of each other.
Answer to Problem 5.37P
The given molecule is not a same enantiomer as the original one.
Explanation of Solution
The given original enantiomer is:

The molecule which is to be compared is:
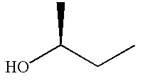
The molecule cannot be converted to the original molecule by single bond rotation.
Hence, the given molecule is not same enantiomer as the original one.
The molecule is not a same enantiomer as original molecule is determined on the basis of capability of interconversion by single bond rotation.
(d)
Interpretation:
The given molecule is same or different enantiomer of the shown original molecule is to be identified.
Concept introduction:
Isomers are molecules having same connectivity. Enantiomers are nonsuperimposable mirror images. If the molecules can be interconverted by one or more single bond rotation, then they are said to be the same enantiomer or identical molecules. If the molecules are not able to interconvert, then they are enantiomers of each other.
Answer to Problem 5.37P
The given molecule is same enantiomer as the original one.
Explanation of Solution
The given original enantiomer is:
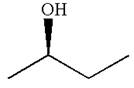
The molecule which is to be compared is:
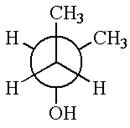
The given Newman projection can be converted to zigzag structure as shown below:
The molecule can be converted to the original molecule.
Hence, the given molecule is same enantiomer as the original one.
The molecule is a same enantiomer as original molecule is determined by converting Newman projection to zigzag structure.
(e)
Interpretation:
The given molecule is same or different enantiomer of the shown original molecule is to be identified.
Concept introduction:
Isomers are molecules having same connectivity. Enantiomers are nonsuperimposable mirror images. If the molecules can be interconverted by one or more single bond rotation, then they are said to be the same enantiomer or identical molecules. If the molecules are not able to interconvert, then they are enantiomers of each other.
Answer to Problem 5.37P
The given molecule is not a same enantiomer as the original one.
Explanation of Solution
The given original enantiomer is:

The molecule which is to be compared is:
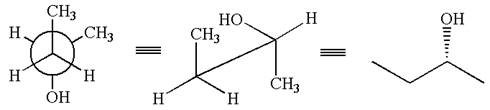
The given Newman projection can be converted to zigzag structure as shown below:
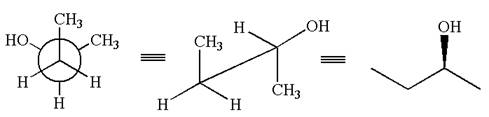
The given molecule is a nonsuperimposable mirror image of the original molecule.
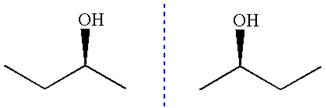
These molecules cannot be interconverted by single bond rotation. Hence, the given molecule is not same enantiomer as the original one.
The molecule is a not the same enantiomer as the original molecule is determined by converting Newman projection to zigzag structure.
(f)
Interpretation:
The given molecule is same or different enantiomer of the shown original molecule it is to be identified.
Concept introduction:
Isomers are molecules having same connectivity. Enantiomers are nonsuperimposable mirror images. If the molecules can be interconverted by one or more single bond rotation, then they are said to be same enantiomer or identical molecules. If the molecules are not able to interconvert, then they are enantiomers of each other. In Fischer projection, the horizontal bonds point towards the observer and are denoted as wedge bond in the zigzag structure.
Answer to Problem 5.37P
The given molecule is a same enantiomer as the original one.
Explanation of Solution
The given original enantiomer is:
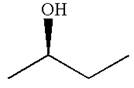
The molecule which is to be compared is:
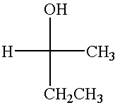
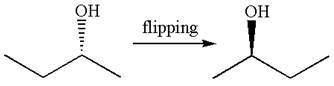
The given Fischer projection can be converted to zigzag structure as shown below:
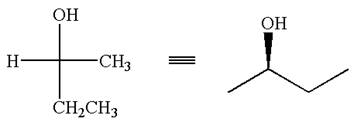
Hence, the given molecule is a same enantiomer as the original one.
The molecule is not a same enantiomer as the original molecule is determined by converting Fischer projection to zigzag structure.
(e)
Interpretation:
The given molecule is same or different enantiomer of the shown original molecule is to be identified.
Concept introduction:
Isomers are molecules having same connectivity. Enantiomers are nonsuperimposable mirror images. If the molecules can be interconverted by one or more single bond rotation, then they are said to be the same enantiomer or identical molecules. If the molecules are not able to interconvert, then they are enantiomers of each other. In Fischer projection, the horizontal bonds point toward the observer and are denoted as wedge bond in the zigzag structure.
Answer to Problem 5.37P
The given molecule is not a same enantiomer as the original one.
Explanation of Solution
The given original enantiomer is:

The molecule which is to be compared is:
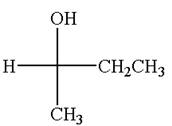
The given Fischer projection can be converted to zigzag structure as shown below:
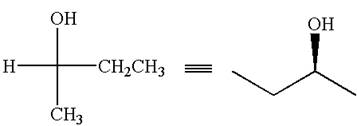
The given molecule is a nonsuperimposable mirror image of the original molecule.
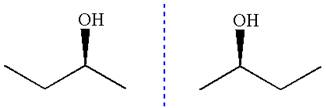
These molecules cannot be interconverted by single bond rotation. Hence, the given molecule is not same enantiomer as the original one.
The molecule is not a same enantiomer as the original molecule is determined by converting Fischer projection to zigzag structure.
(g)
Interpretation:
The given molecule is same or different enantiomer of the shown original molecule it is to be identified.
Concept introduction:
Isomers are the molecules having same connectivity. Enantiomers are nonsuperimposable mirror images. If the molecules can be interconverted by one or more single bond rotation, then they are said to be the same enantiomer or identical molecules. If the molecules are not able to interconvert, then they are enantiomers of each other.
Answer to Problem 5.37P
The given molecule is not a same enantiomer as the original one.
Explanation of Solution
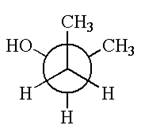
The given original enantiomer is:

The molecule which is to be compared is:
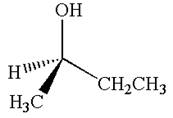
This molecule can be converted to original molecule by single bond rotation.
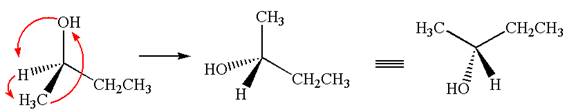
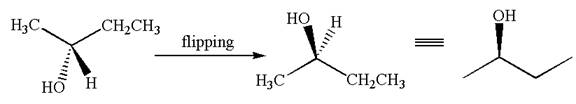
Hence, the given molecule is same enantiomer as the original one.
The molecule is a same enantiomer as the original molecule is determined on the basis of capability of interconversion by single bond rotation.
Want to see more full solutions like this?
Chapter 5 Solutions
Organic Chemistry: Principles and Mechanisms (Second Edition)
- Determine the R/S configuration of each chiral center in the molecules shown below. (Do not include lone pairs as an attachment when identifying the chiral centers.)arrow_forwardCheck the box under each structure in the table that is an enantiomer of the molecule shown below. If none of them are, check the none of the above box under the table. Br HI!... OH Molecule 1 *** H" Br H Molecule 4 OH Br OH Onone of the above Molecule 2 H Br Br ""OH ОН Molecule 5 OH _ H Molecule 3 Br CI H Molecule 6 Br H OH X 5arrow_forwardCircle all of the chiral centers (i.e., asymmetric carbon atoms) in the following compounds.arrow_forward
- Check the box under each structure in the table that is an enantiomer of the molecule shown below. If none of them are, check the none of the above box under the table. * Molecule 1 Molecule 4 q Onone of the above Molecule 2 Molecule 5 Molecule 3 Molecule 6 *** X 0arrow_forwardHow many stereoisomers does the molecule shown below have? Draw them.arrow_forwardDoes the molecule below exist as a pair of enantiomers? If so, change the bonds to wedges and dashes to reflect R stereochemistry. If the molecule does not exist as a pair of enantiomers, check the box below.arrow_forward
- 3. Assign R or S stereochemistry to each of the chiral centers in the molecules below. OH H3C NH2 OH Br CI CH3 OH Br Br Br HO H Harrow_forwardClick on all structures that are enantiomers of the first (leftmost). If no structure qualifies, submit your answer without selecting any structure. CH3 CH3 H CH2B HO HO H3C HO CH2BR H. BrH2C H. CH2BR CH3 ноarrow_forwardCheck the box under each structure in the table that is an enantiomer of the molecule shown below. If none of them are, check the none of the above box under the table. H" CI OH Molecule 1 HO H H 0 Molecule 4 CI OH Br none of the above Molecule 2 H OH Br Molecule 5 НО HO*** H Br Molecule 3 H Br HO Molecule 6 H OH ליי CI X Sarrow_forward
- Which of the following structures is the correct wedge/dash drawing of the following Fischer projection? H Br- Et Br -Br H Br || ||| IV Br Br +x Br Br ||| Br IVarrow_forwardQuestion 3: Please state whether each of the following molecules is chiral or achiral. If you believe it is achiral, indicate the element of symmetry [plane of symmetry or center of inversion] responsible for the achirality. H3C H3C |H3C OH Br Br -H H CI Br но H Н CI CI CI ОН НН ОН H CI ОН ОН Br- Br H3C. .H неи Н' С2Н5 Н CI CI Br >arrow_forwardClick on all structures that are enantiomers of the first (leftmost). If no structure qualifies, submit your answer without selecting any structure. OCH3 CI Br H3CO F Br Br Br CI F CI H3CO H;COarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





