
Concept explainers
To review:
The classification of the following amino acids according to theirstructuresas nonpolar, polar, acidic, or basic.
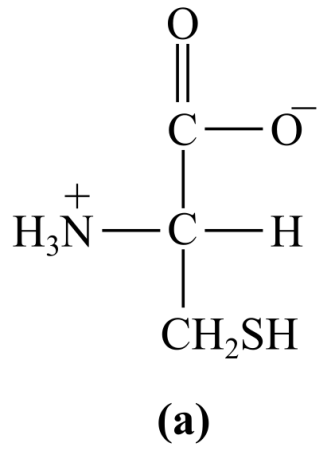
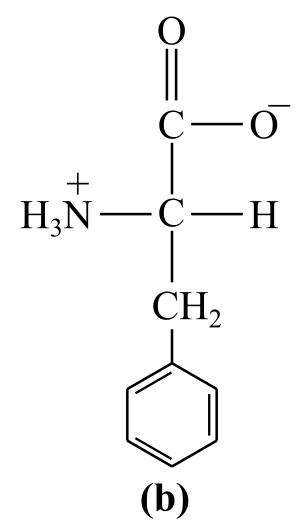
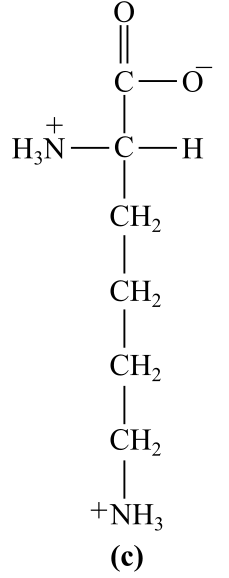
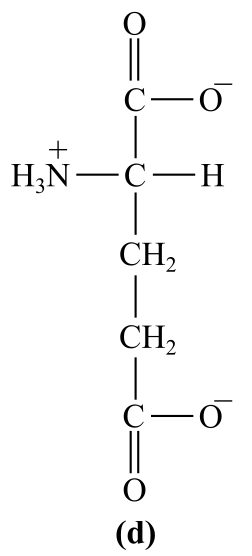
Introduction:
Amino acids are the basic unit of a protein. The chain of an amino acid is calledpolypeptide. Each amino acid has a carboxylic end and an amino end. One amino acid is linked to another via a peptide bond. There are 20 amino acids that are found naturally in our body and are known as standard amino acids.
Explanation of Solution
The amino acids can be classified as polar, nonpolar, acidic, and basic. The nonpolar amino acids have hydrocarbonscontaining R group (side chain), and they lack any type of charge. The polar amino acids have a polar hydroxyl group (-OH). The -SH group (sulfhydryl group) is also polar in nature. The acidic amino acids have R chain containing carboxylate group (-COOH), while the basic amino acid has an amino group in the side chain.
The structure (a) in the given question is the amino acid, cysteine. It has a polar group of -SH in its R-chain (side chain). Thus, it is a polar amino acid.
The structure (b) in the given question is the amino acid, phenylalanine. It has a nonpolar group:a benzene ring, as its side chain, which bear no positive or negative charges. Thus, it is a nonpolar amino acid.
The structure (c) in the given question is the amino acid, lysine. The amino group is present in the R-chain, which bears positive charge at physiological pH, which is basic in nature. Thus, it is a basic amino acid.
The structure (d) in the given question is the amino acid, glutamate. It has a carboxylic group as the R-chain, which bears negative charge at physiological pH, which is acidic in nature.
Therefore, it can be concluded that the given structures are classified as
a) Polar (cysteine)
b) Nonpolar (phenylalanine)
c) Basic (lysine)
d)Acidic (glutamate)
Want to see more full solutions like this?
Chapter 5 Solutions
Biochemistry: The Molecular Basis of Life
- Mark as true or false the statements about amino acid reactivity and peptide synthesis: ( ) Peptides are very diverse in terms of their function/application. Some are hormones or their releasing factors, some are neurotransmitters, some are toxins, some are natural antibiotics, and some work as sweeteners, such as aspartame. ( ) Most of the natural peptides occur in low concentration, which makes their isolation from the matrix difficult, and therefore, their chemical synthesis is necessary. ( ) The chemical synthesis of peptides is only done with the use of enzymes ( ) A difficulty in the synthesis of peptides is due to the fact that carboxylic acids and primary or secondary amines do not form amide bonds easily. When mixing the two, what we would have would be products from the transfer of protons between them, forming a charged species. ( ) The chemical synthesis of peptides uses a chemical reagent to activate the carboxylic acid of the amino acid, which will undergo nucleophilic…arrow_forwardNon-standard amino acids are derived from common amino acids by biochemical modifications. Please identify the chiral centers of given non-standard amino acids.arrow_forwardFor the amino acids listed below, what tertiary/quaternary interaction can each of the amino acids participate in? 1)Alanine 2)Asparagine 3) Aspartic Acid 4) Argininearrow_forward
- Determine the amino acids that compose the peptide shown below.arrow_forwardClassify these amino acids as acidic, basic, neutral polar, or neutral nonpolar. Glutaminc Acid, Threonine, Tyrosine, Valine, and tryptophanarrow_forwardDescribe citrulline and ornithine based on their similarityto one of the 20 standard amino acids.arrow_forward
- Discuss the classification of amino acids.arrow_forwardIn each of the following pairs of amino acids, identify which amino acid would be more soluble in water: (a) Ala, Leu; (b) Tyr, Phe; (c) Ser, Ala; (d) Trp, Hisarrow_forwardWhy is it important to specify the three-dimensional structure of amino acids?arrow_forward
- g) Describe the biological function of the peptide hormone insulin. h) Haemoglobin is an example of a quaternary protein. Briefly describe the structure of this protein and state its biological function.arrow_forwardWrite down the abbreviations (both 1 letter and 3 letter) for the amino acids given below:Tryptophan, Glutamine, Isoleucine, Cysteine, Argininearrow_forwardWhat is the predominant form of each of the following amino acids at pH = 11? What is the overall charge on the amino acid? (a) valine; (b) proline; (c) glutamic acid; (d) lysinearrow_forward
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON





