
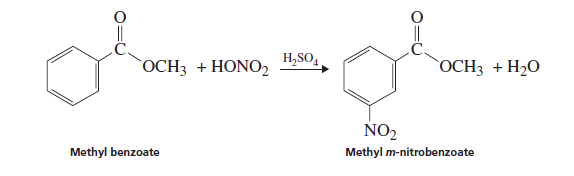
Interpretation: The reason for the formation of methyl m -nitrobenzoate instead of ortho and para isomers in the following reaction needs to be explained:
Concept Introduction: The organic reactions where an atom which is attached to an
Answer to Problem 1Q
Due to the presence of an electron-withdrawing group that is ester group (
Explanation of Solution
The methyl benzoate is less reactive towards aromatic electrophilic substitution due to the presence of an electron-withdrawing group that is the ester group,
Due to the -R effect (The withdrawal of electrons from one molecule by the other group in a molecule through delocalization is said to be a negative resonance effect (-R effect)), the
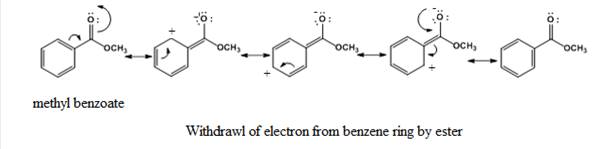
The partial positive charge is present at ortho and para position on the benzene ring and thus, it will deactivate the benzene ring towards electrophilic substitution reactions. The incoming electrophile will attack on a position where the electron density is high that is at the meta position.
Want to see more full solutions like this?
Chapter 43 Solutions
EBK A SMALL SCALE APPROACH TO ORGANIC L
- What is the major product of nitration of p-tert-butylmethylbenzene reaction?arrow_forwardExplain why pentane-2,4-dione forms two different alkylation products (A or B) when the number of equivalents of base is increased from one to two.arrow_forwardBased on the image attached, explain the step-by-step mechanism reaction of nitration for methyl salicylate that formed 3-Nitrosalicylate as a product.arrow_forward
- What change can be made to allow for a faster rate of reaction if poor solubility of KOH in ethanol slowed the rate and led to slow deprotonation of dibenzyl ketone?arrow_forwardThe coupling of an alkyne with an aryl halide in the presence of a palladium catalyst and triethylamine is called a Sonogashira reaction. What reactants couple to form this product?arrow_forwardWhat is the correct reaction scheme look like between acetic acid and 3-methylbutanol in the presence of sulfuric acid? Which image attached is the correct reaction scheme?arrow_forward
- Which of these is a transition stage in ethene hydrohalogenation?arrow_forwardOChem 2 in the attached image, identify which compounds would exist in the ENOL form primarily? Draw the tautomer of the chosen one and explain why this is.arrow_forwardExplain why methyl trifluoroacetate, CF3CO2CH3, is more reactive than methyl acetate, CH3CO2CH3, in nucleophilic acyl substitution reactions.arrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

